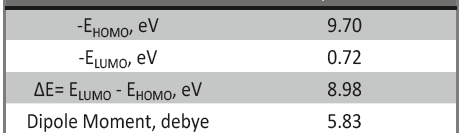
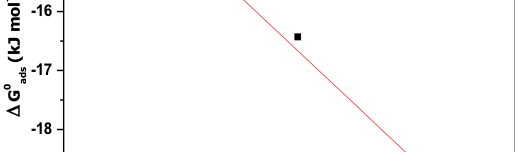
The corrosion inhibition of mild steel in 0.5 M H2SO4 solution by pharmaceutical antibacterial drug named Cephalexin has been investigated by using weight loss, potentiodynamic polarization, electrochemical frequency modulation technique (EFM) and electrochemical impedance spectroscopy (EIS) measurements. The polarization data showed that this drug is mixed-type inhibitor. The percentage inhibition efficiency was found to increase with increasing the concentration of the drug and with decreasing temperature. The Langmuir`s isotherm was found to provide an accurate description of adsorption behavior of this drug. Some thermodynamic parameters were computed and discussed. The correlations between advanced quantum chemical concepts and inhibition efficiency was found and discussed. The data obtained from different methods are in good agreement.
Corrosion protection of 6061 Al-15 Vol. Pct. SiC(p) composite using a biopolymer- An electrochemical approach
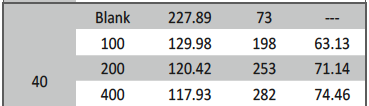
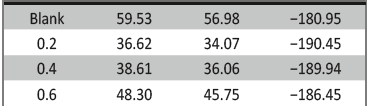
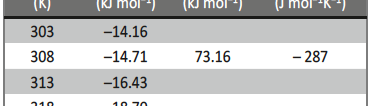
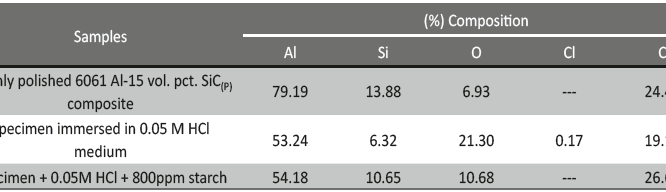
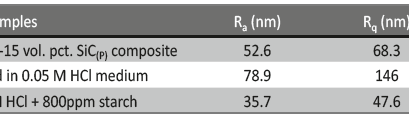
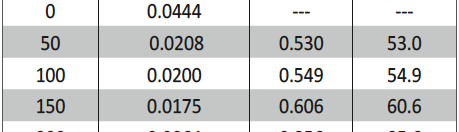
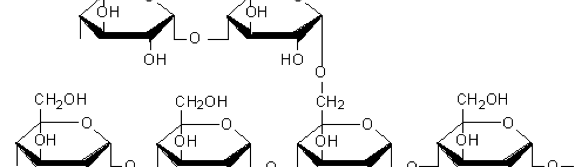
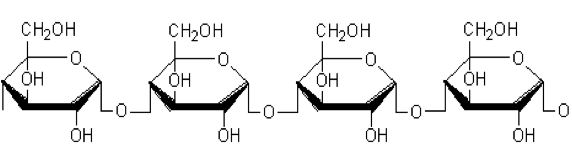
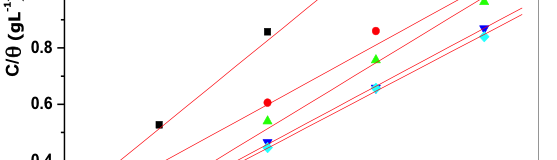
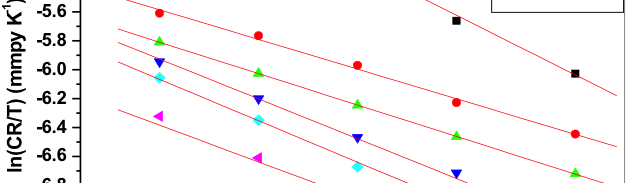
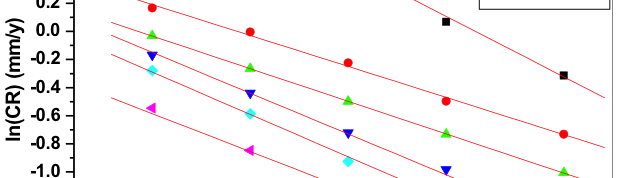
The influence of biopolymer starch as corrosion inhibitor on 6061 Al-15 vol. pct. SiC(p) composite in 0.05M hydrochloric acid was studied by potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) technique. The surface morphology was studied using SEM, EDX, AFM and XRD techniques. The results showed that the inhibition efficiency of starch increased with increasing inhibitor concentrations and also with increase in temperatures. Starch acted as a mixed inhibitor and underwent chemical adsorption following Langmuir adsorption isotherm.
Prof. Isabel Díaz Tang – New member of the Advisory Board
A few days ago we had introduced to you Dr. Saeid Kakooei as an new member of our Advisory Board. Today I have the great pleasure to welcome Prof. Isabel Díaz Tang too. Continue reading…
Dr. Saeid Kakooei - New member of the Advisory Board
Today we want to introduce Dr. Saeid Kakooei to you. He is a new member of our Advisory Board. Working as a lecturer he is teaching Corrosion Engineering, Engineering Materials and Thermodynamic courses in Universiti Teknologi Petronas (UTP) to Undergraduate students of Mechanical Engineering Department, he has a lot of experience. Continue reading…
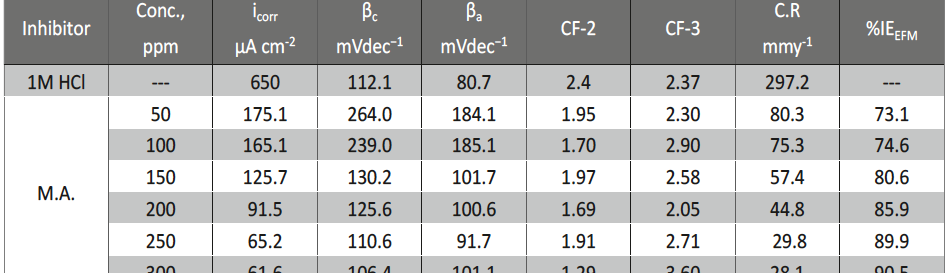
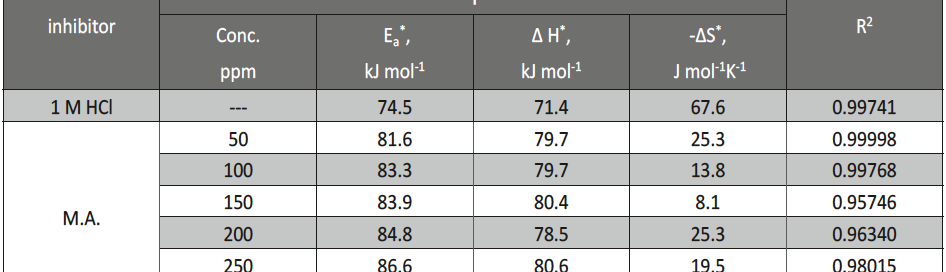
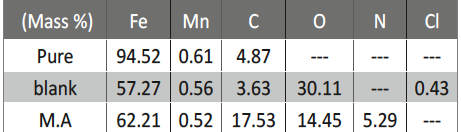
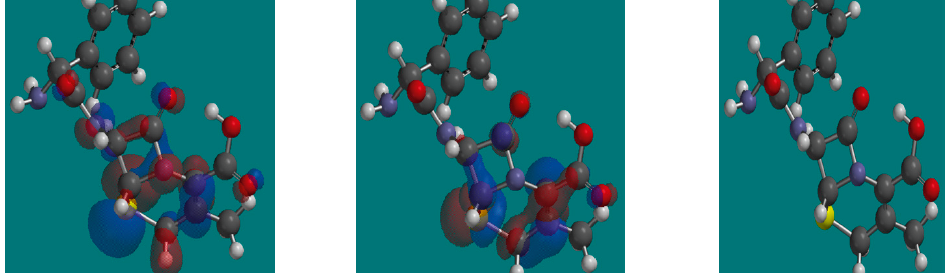
Malonic Acid as Corrosion Inhibitor for Carbon Steel in 1 M Hydrochloric Acid Solutions
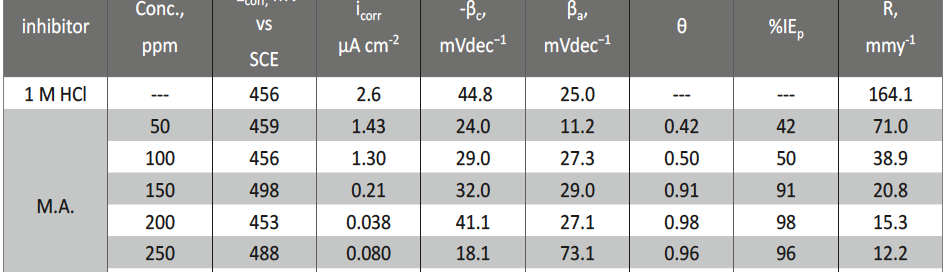
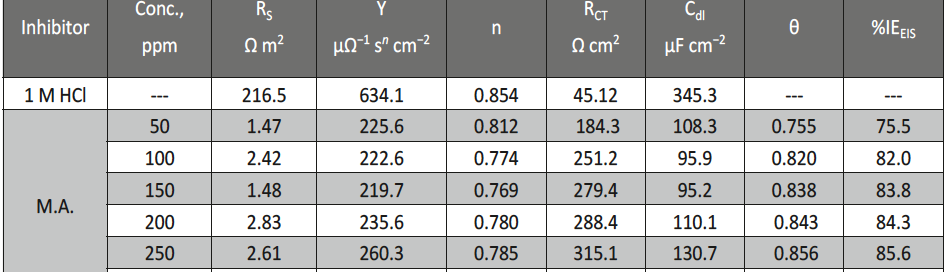
The protection effect of malonic acid on carbon steel corrosion was studied in aerated stagnant 1M HCl solutions at 250C. Measurements were conducted under different experimental conditions using weight loss, Tafel polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. malonic acid was found to be good inhibitor of carbon steel corrosion in1 M HCl. The adsorption of this inhibitor is found to obey the Langmuir adsorption isotherm. The calculated activation energies proposed that the inhibitor molecules being physically adsorbed onto the metal surface. Polarization data revealed that this compound behave as mixed type inhibitor.


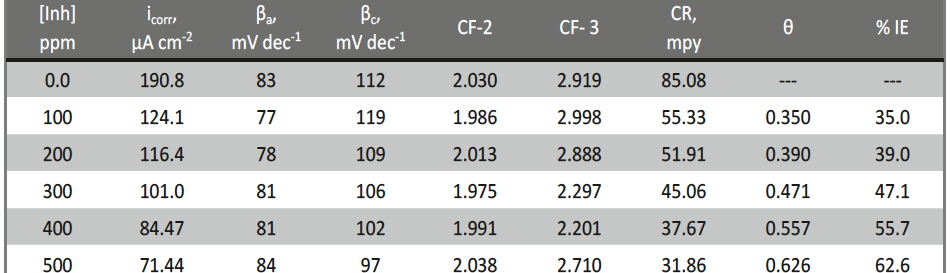
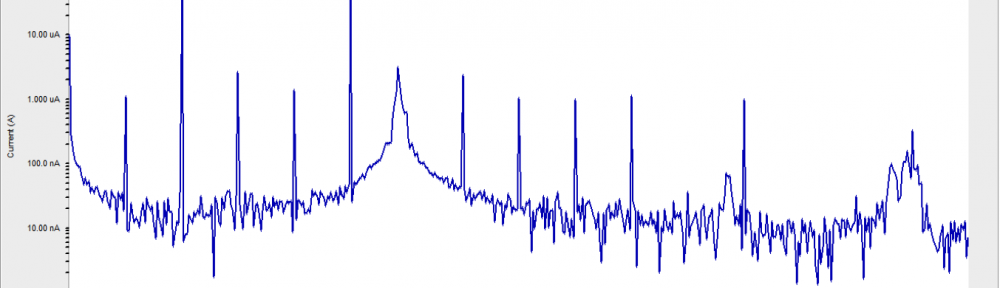
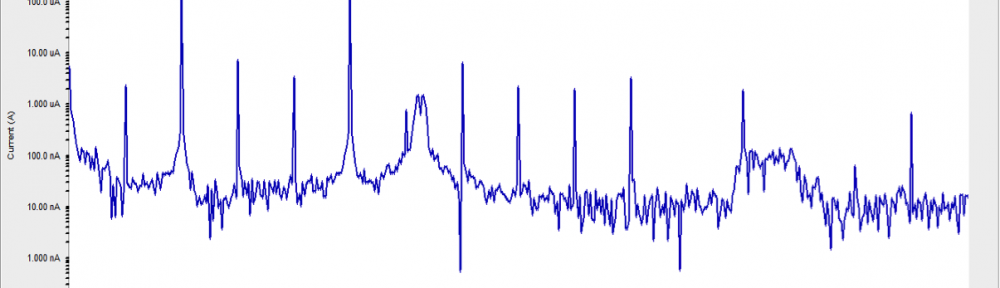
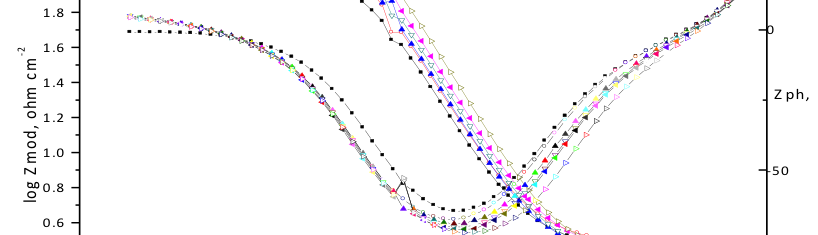
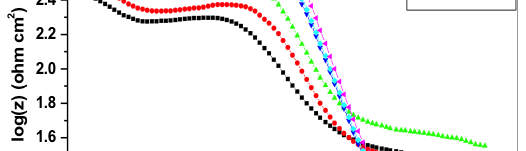
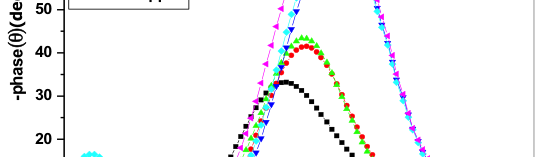
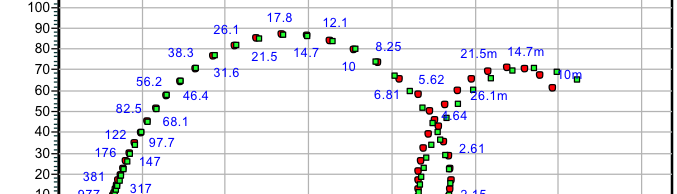
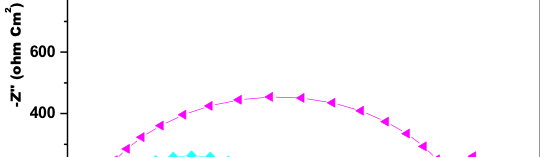
![Fig. 2: Potentiodynamic polarization curves for the corrosion of 6061 Al-15 vol. pct. SiC(P) composite in 0.05M HCl containing various [starch] at 35 °C](https://www.jept.de/wp-content/uploads/2016/02/pic2-578x166.png)
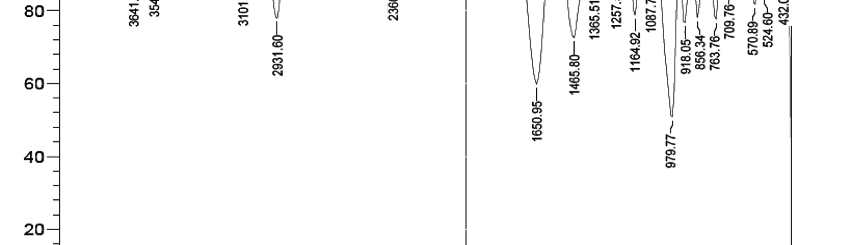







![Tab. 2: Results of Potentiodynamic polarization studies for corrosion of 6061 Al-15 vol. pct. SiC(P) composite in 0.05M HCl containing various [starch]](https://www.jept.de/wp-content/uploads/2016/02/tab2-756x217.png)