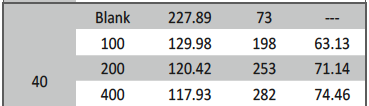
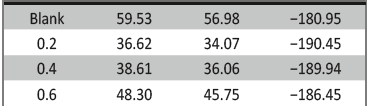
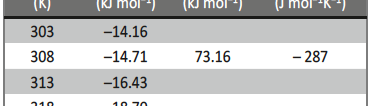
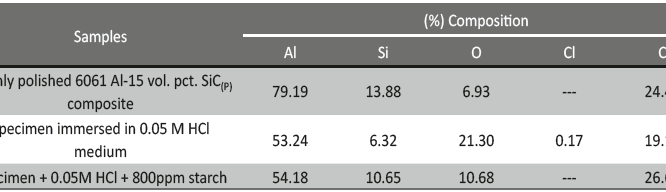
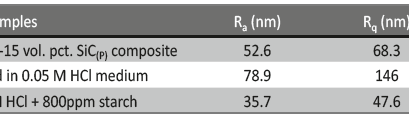
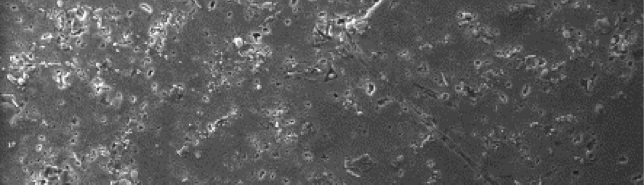
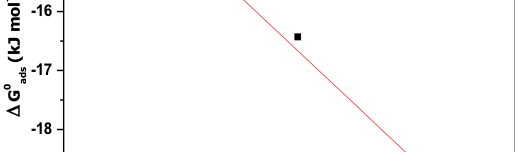
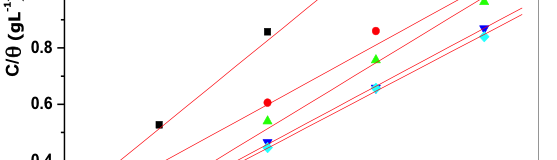
The influence of biopolymer starch as corrosion inhibitor on 6061 Al-15 vol. pct. SiC(p) composite in 0.05M hydrochloric acid was studied by potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) technique. The surface morphology was studied using SEM, EDX, AFM and XRD techniques. The results showed that the inhibition efficiency of starch increased with increasing inhibitor concentrations and also with increase in temperatures. Starch acted as a mixed inhibitor and underwent chemical adsorption following Langmuir adsorption isotherm.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany

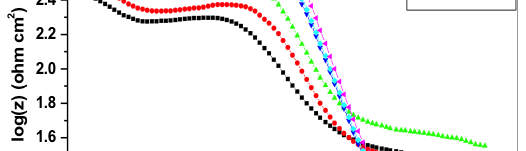
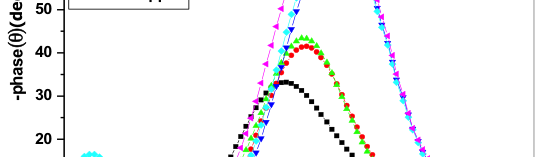
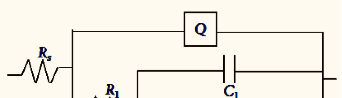
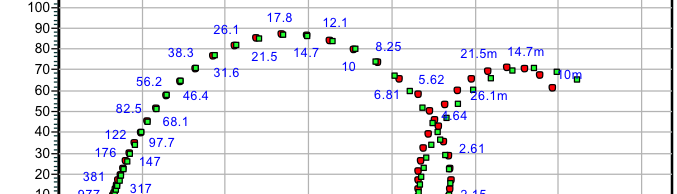
![Fig. 2: Potentiodynamic polarization curves for the corrosion of 6061 Al-15 vol. pct. SiC(P) composite in 0.05M HCl containing various [starch] at 35 °C](https://www.jept.de/wp-content/uploads/2016/02/pic2-578x166.png)
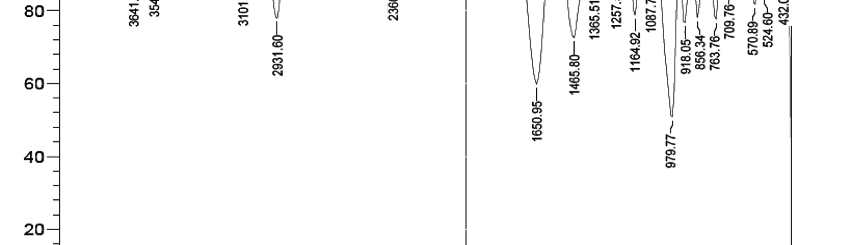







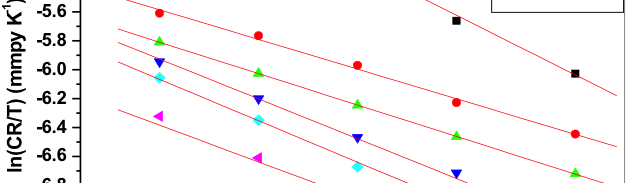
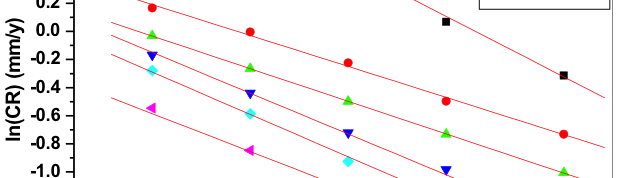
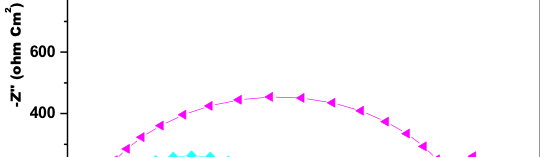
![Tab. 2: Results of Potentiodynamic polarization studies for corrosion of 6061 Al-15 vol. pct. SiC(P) composite in 0.05M HCl containing various [starch]](https://www.jept.de/wp-content/uploads/2016/02/tab2-756x217.png)