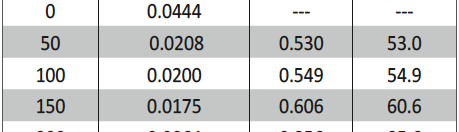
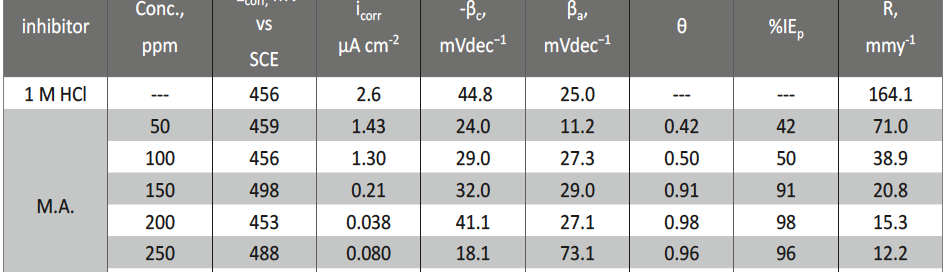
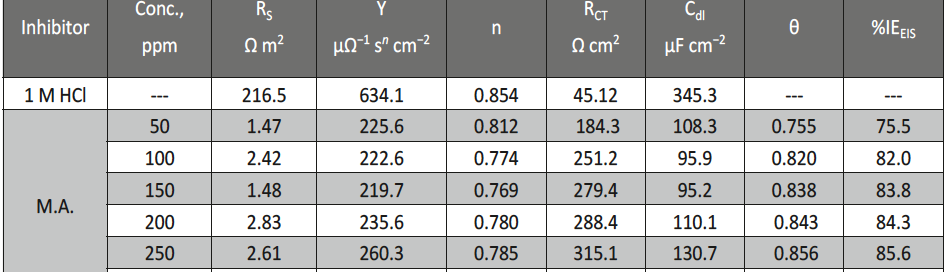
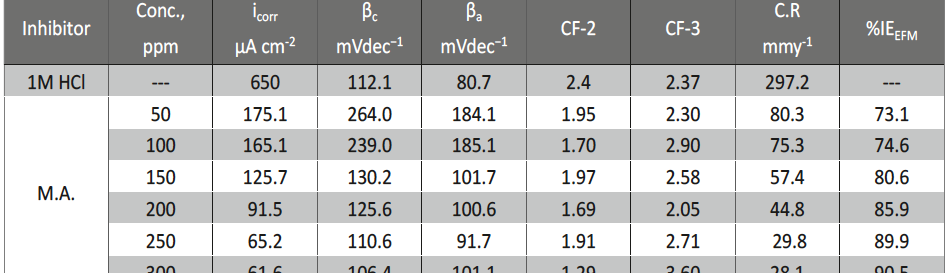
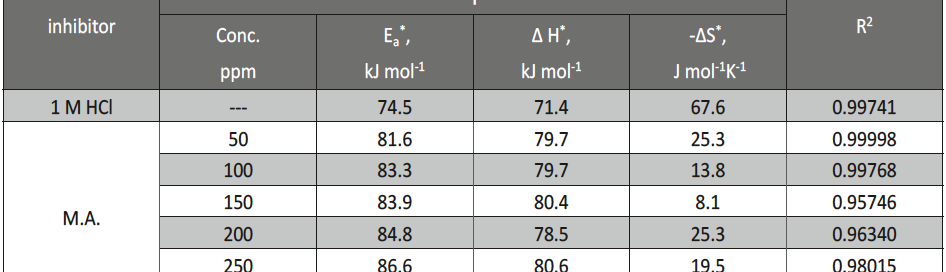
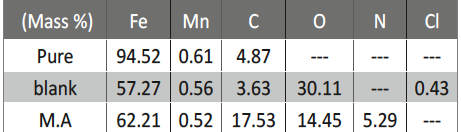
The protection effect of malonic acid on carbon steel corrosion was studied in aerated stagnant 1M HCl solutions at 250C. Measurements were conducted under different experimental conditions using weight loss, Tafel polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. malonic acid was found to be good inhibitor of carbon steel corrosion in1 M HCl. The adsorption of this inhibitor is found to obey the Langmuir adsorption isotherm. The calculated activation energies proposed that the inhibitor molecules being physically adsorbed onto the metal surface. Polarization data revealed that this compound behave as mixed type inhibitor.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany