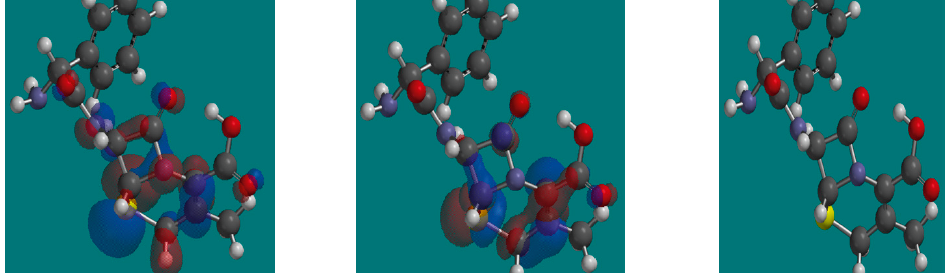
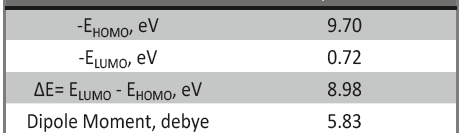
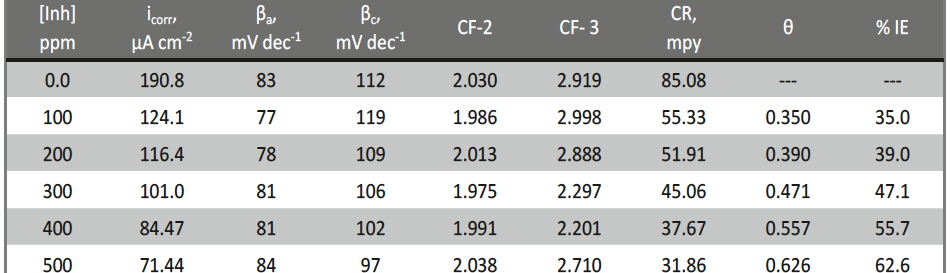
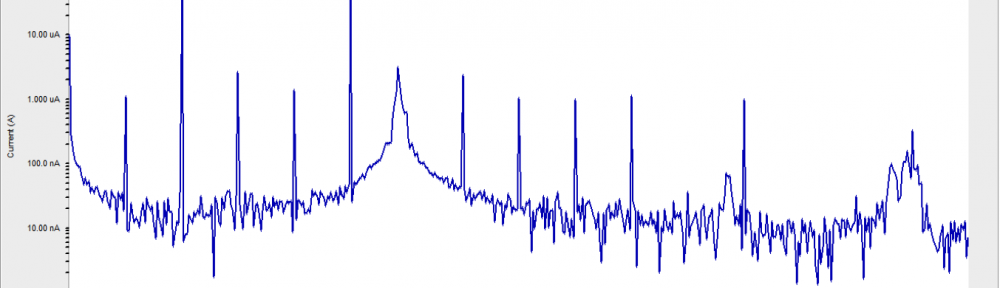
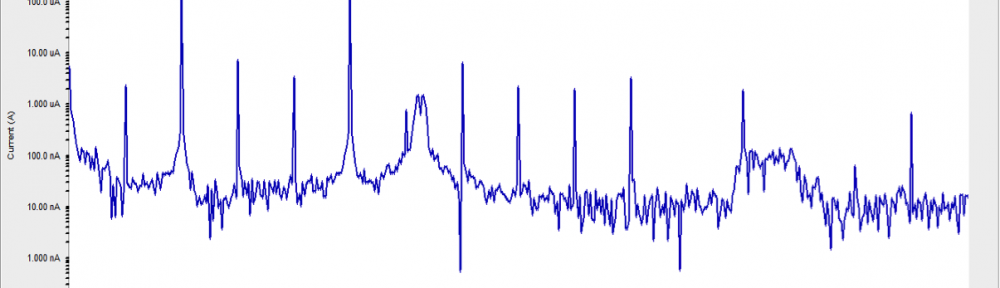
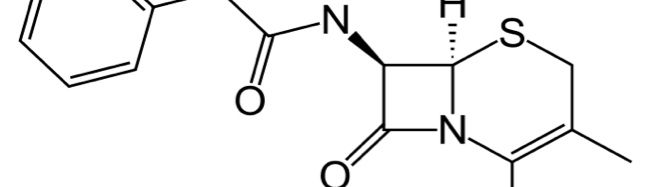
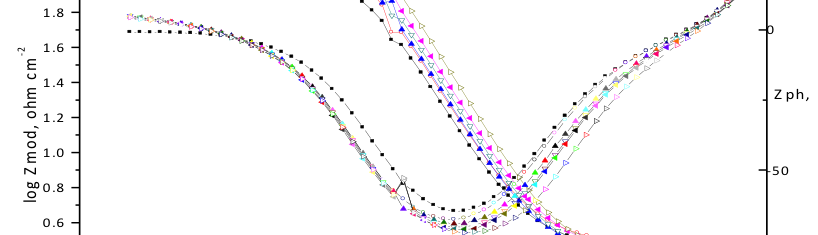
The corrosion inhibition of mild steel in 0.5 M H2SO4 solution by pharmaceutical antibacterial drug named Cephalexin has been investigated by using weight loss, potentiodynamic polarization, electrochemical frequency modulation technique (EFM) and electrochemical impedance spectroscopy (EIS) measurements. The polarization data showed that this drug is mixed-type inhibitor. The percentage inhibition efficiency was found to increase with increasing the concentration of the drug and with decreasing temperature. The Langmuir`s isotherm was found to provide an accurate description of adsorption behavior of this drug. Some thermodynamic parameters were computed and discussed. The correlations between advanced quantum chemical concepts and inhibition efficiency was found and discussed. The data obtained from different methods are in good agreement.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany