Environmentally friendlier and safer alternatives are required as replacement for highly toxic cyanide baths used by electroplating industries. Glutamate has proved to be an adequate complexing agent in alkaline copper and zinc plating baths due to its capability to form anionic complexes with those metal ions. Consequently, studies with a glutamate-based electrolyte for Cu-Zn alloys deposition are presented.
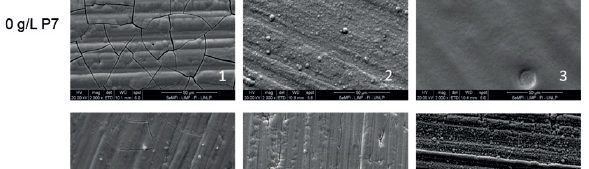
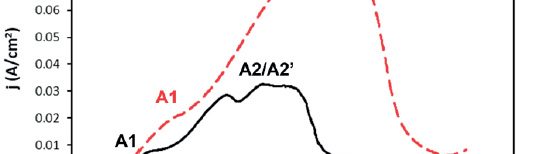
In this work, cathodic polarization experiments on a rotating disk electrode in both static and rotating conditions were carried out using the novel electrolyte with and without the addition of a polymeric cationic surfactant (Polyquaternium-7). The deposits were later dissolved by anodic stripping to characterize the electrochemical processes involved in the Cu-Zn-Glutamate system. Galvanostatic experiments, using flat steel electrodes as substrate, were carried out at different current densities with and without additive. These coatings were characterised by SEM, EDS and XRD.
Cu-Zn alloys with compositions between 37-83 wt.% of copper were obtained. α, β and γ phases were obtained depending on the electrolyte composition and the applied current density.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany