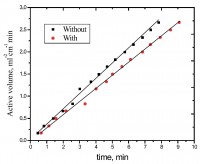
The influence of water-soluble poly (vinyl alcohol) (PVA) as a synthetic polymer containing secondary alcoholic groups on the rate of dissolution of aluminum (Al) metal in alkaline medium has been investigated using both gasometric and weight-loss techniques. The results showed that addition of poly (vinyl alcohol) to the tested solutions leads to a remarkable decrease in the corrosion rates of Al in alkali. The magnitude of inhibition efficiency was determined and compared to that obtained with other macromolecules containing secondary alcoholic groups. The inhibition action of PVA on Al metal surface was found to obey Freundlich adsorption isotherm. Factors affecting the corrosion process such as the concentration and nature of the inhibitor, concentration of the corrosive medium and the temperature were examined. A tentative inhibition mechanism consistent with the kinetic results obtained is suggested and discussed.
