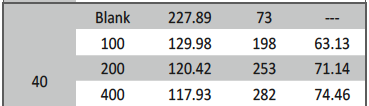
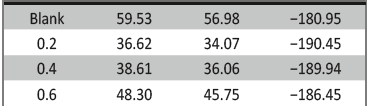
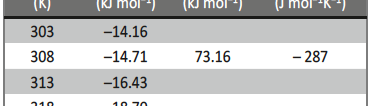
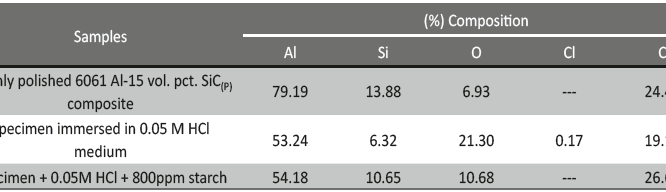
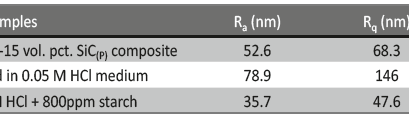
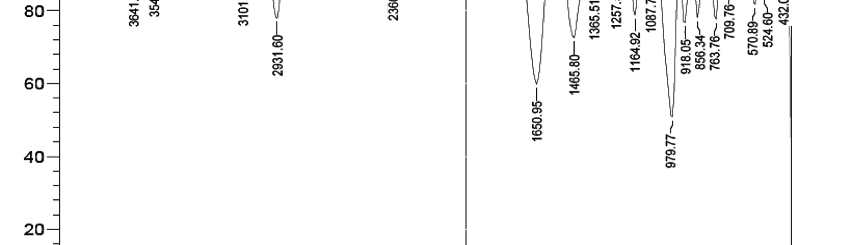
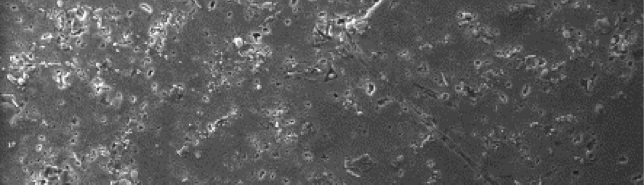
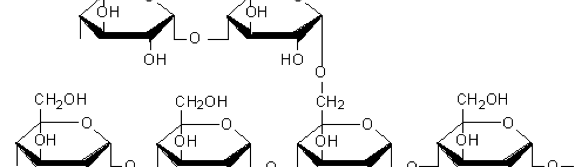
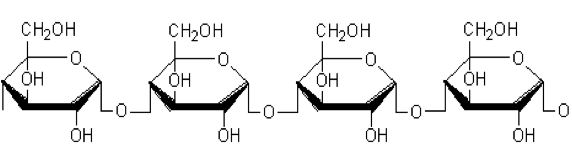
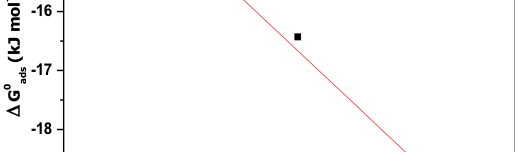
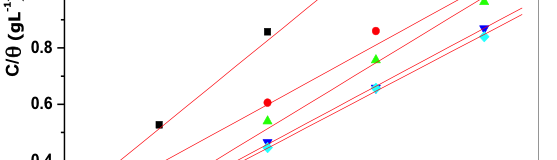
The influence of biopolymer starch as corrosion inhibitor on 6061 Al-15 vol. pct. SiC(p) composite in 0.05M hydrochloric acid was studied by potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) technique. The surface morphology was studied using SEM, EDX, AFM and XRD techniques. The results showed that the inhibition efficiency of starch increased with increasing inhibitor concentrations and also with increase in temperatures. Starch acted as a mixed inhibitor and underwent chemical adsorption following Langmuir adsorption isotherm.
Tag Archives: EIS
Adsorption and Corrosion Inhibition Characteristics of Some Thiophene-3-Carbohydrazide Derivatives on Low Carbon Steel in Hydrochloric Acid Solutions
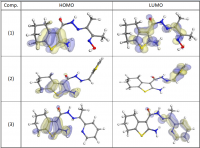
New compounds of corrosion inhibitors namely amino-N’-(3-(hydroxyimino)butan-2-ylidene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (1), amino-N’-(thiophen-2-ylmethylene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (2) and amino-N’-(1-(pyridin-2-yl)ethylidene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (3) were synthesized and its inhibiting action on the corrosion of carbon steel in 1 M hydrochloric acid at 25ºC was investigated by various corrosion monitoring techniques. A Potentiodynamic polarization, AC impedance and electrochemical frequency modulation methods have been used. Potentiodynamic polarization studies showed that these derivatives were mixed type inhibitors. The effect of temperature on the corrosion behavior of carbon steel in 1 M HCl with the addition of these compounds were studied in the temperatures 25 and 45ºC. The adsorption of these inhibitors on carbon steel surface from hydrochloric acid obeyed the Langmuir adsorption isotherm. Quantum chemical method is used to explore the relationship between the inhibitors molecular properties and their inhibition efficiency.
Effect of 0.5wt% Cu Content on the Electrochemical Corrosion of Heat Treated Al-6Si-0.5Mg Alloy in Simulated Seawater

Al-Si hypoeutectic alloys produced by casting are mostly used in the automotive industry, especially for engine blocks. They have the advantage of low weight associated with low coefficient of thermal expansion and excellent mechanical properties. The corrosion resistance of these alloys in coastal area, particularly in seawater environment is not well known. In this investigation, electrochemical impedance spectroscopy (EIS) and potentiodynamic polarisation have been used to evaluate the corrosion resistance of Cu free and 0.5wt% Cu content Al-6Si-0.5Mg alloy in simulated seawater environment. The 0.5wt% Cu addition to the Al-6Si-0.5Mg alloy showed that Cu decreased susceptibility to electrochemical corrosion compared to the Cu free Al-6Si-0.5Mg alloy. The magnitude of open circuit potential (OCP), corrosion potential (Ecorr) and pitting corrosion potential (Epit) of Al-6Si-0.5Mg alloy in simulated seawater were shifted to the more noble direction due to 0.5wt% Cu addition and thermal modification.
Electrochemical Investigation on the Corrosion Behavior of Combined Addition of Cu and Ni to Al-Si-Mg Alloy in 0.1M NaCl Solution

The purpose of this paper is to understand the effect of 2Cu+2Ni addition on electrochemical corrosion behavior of thermal treated Al-6Si-0.5Mg alloy in 0.1M NaCl solution. The corrosion of the thermal treated samples was characterized by electrochemical potentiodynamic polarization technique consisting of linear polarization method using the fit Tafel plot and electrochemical impedance spectroscopy (EIS) techniques. Generally, from the linear polarization, the corrosion rate decreases at thermal treated Al-6Si-0.5Mg-2Cu-2Ni alloy (Alloy-2). The corrosion behavior of the Alloy-2 in the 0.1M NaCl solution showed better resistance than the Alloy-1. The EIS test results also showed that the changing of charge transfer resistance (Rct) is significant with the combined addition of 2Cu+2Ni to Al-6Si-0.5Mg alloy. The magnitude of the noble shift in the open circuit potential (OCP), corrosion potential (Ecorr) and pitting corrosion potential (Epit) increased with the addition of 2Cu+2Ni to Al-6Si-0.5Mg alloy.
Electrochemical Investigation on the Corrosion Behaviour of Mg-Al-Zn-Mn (GA9) Alloy in Sodium Chloride Medium
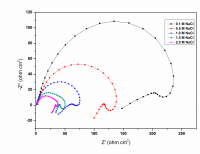
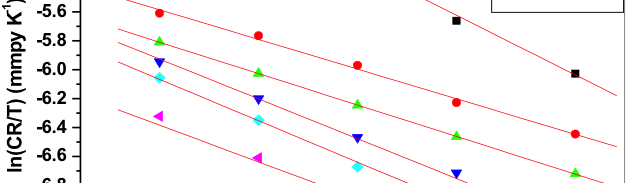
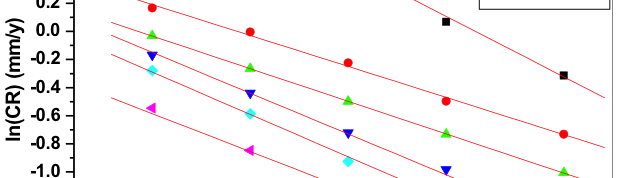
The corrosion behavior of Mg-Al-Zn-Mn (GA9) alloy in sodium chloride solutions was studied over a range of concentrations and solution temperatures by electrochemical techniques like potentiodynamic polarization (PP) and electrochemical impedance spectroscopy (EIS). The studies were carried out in solutions with NaCl of concentrations between 0.1M – 2M; and at different temperatures in the range of 30 ᴼC – 50 ᴼC. The studies have revealed that the corrosion rate of GA9 magnesium alloy increases with the increase in temperature and also with the increase of NaCl concentration in the medium. Activation parameters like activation energy, enthalpy of activation and entropy of activation for evaluation of the corrosion process were calculated. The results from both the techniques are in good agreement with each other. The alloy surface morphology was studied before and after corrosion using scanning electron microscopy (SEM).

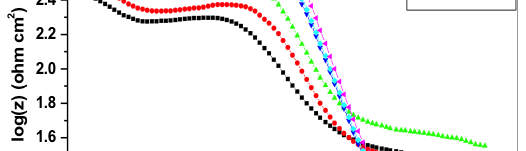
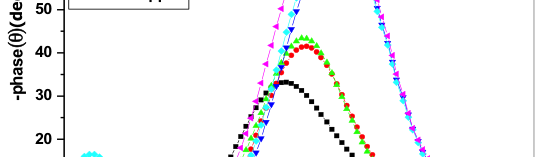
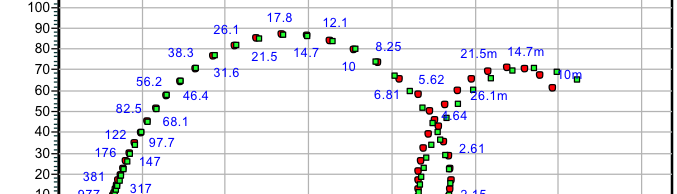
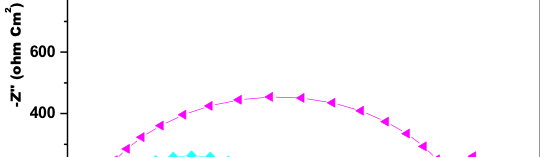
![Fig. 2: Potentiodynamic polarization curves for the corrosion of 6061 Al-15 vol. pct. SiC(P) composite in 0.05M HCl containing various [starch] at 35 °C](https://www.jept.de/wp-content/uploads/2016/02/pic2-578x166.png)







![Tab. 2: Results of Potentiodynamic polarization studies for corrosion of 6061 Al-15 vol. pct. SiC(P) composite in 0.05M HCl containing various [starch]](https://www.jept.de/wp-content/uploads/2016/02/tab2-756x217.png)