A Study on Environmentally Friendly Electroless-Plating
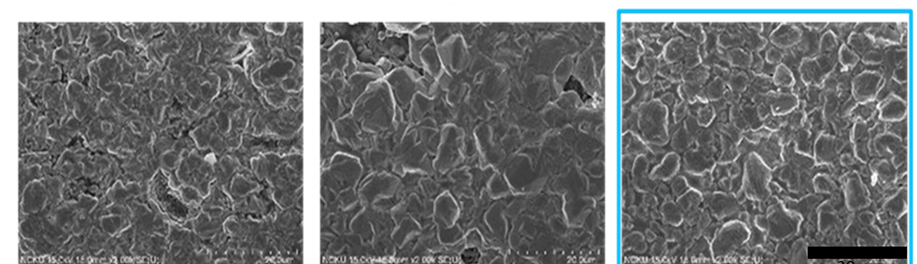
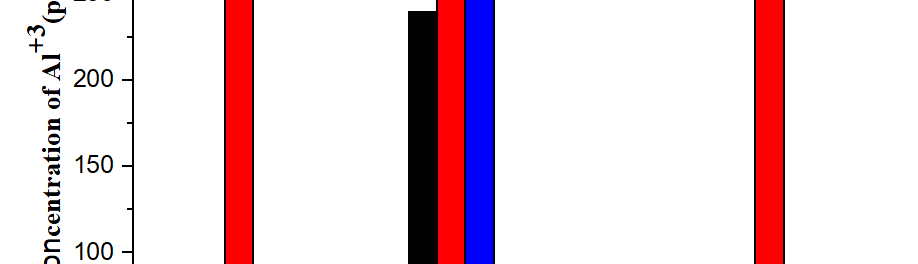
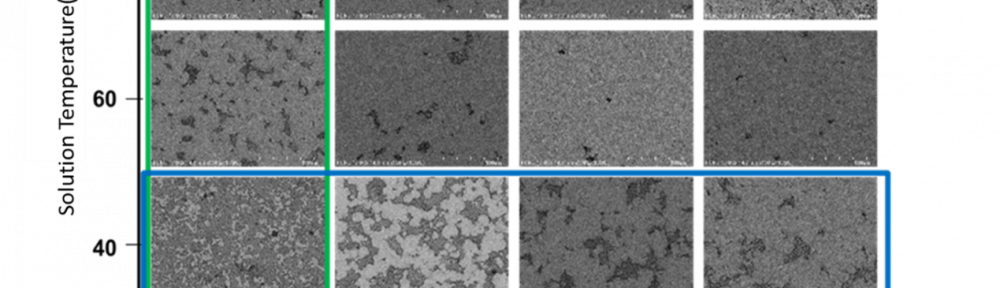
An innovative environmentally friendly electroless plating technology is proposed in this research. This innovative technology, which is a modified galvanic displacement electroless plating technology, combines a thick film aluminum conductor with the galvanic redox chemical displacement reaction. The thick film Al conductor, which has three major characteristics, including a high oxidation potential, a porous structure, and ultra-low cost, is used to define a pattern by using screen printing, where a sacrificial layer is used to carry out the galvanic displacement reaction with other metal ions. Since the existing displacement electroless plating is only reacted on the surface of metal, a porous, thick film Al conductor is used instead of a metal film to obtain entire replacement when the galvanic redox displacement reaction is carried out with low oxidation potential metal ions, such as copper, nickel, etc. In this study, the innovative environmentally friendly displacement electroless plating technique solves the environmental issues associated with electroless plating of Cu with formaldehyde and electroless plating of Ni with lead, and thus provides clean, electroless plating of Cu and Ni films with good electrical performance.