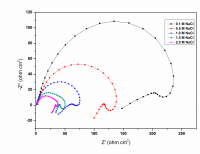
The corrosion behavior of Mg-Al-Zn-Mn (GA9) alloy in sodium chloride solutions was studied over a range of concentrations and solution temperatures by electrochemical techniques like potentiodynamic polarization (PP) and electrochemical impedance spectroscopy (EIS). The studies were carried out in solutions with NaCl of concentrations between 0.1M – 2M; and at different temperatures in the range of 30 ᴼC – 50 ᴼC. The studies have revealed that the corrosion rate of GA9 magnesium alloy increases with the increase in temperature and also with the increase of NaCl concentration in the medium. Activation parameters like activation energy, enthalpy of activation and entropy of activation for evaluation of the corrosion process were calculated. The results from both the techniques are in good agreement with each other. The alloy surface morphology was studied before and after corrosion using scanning electron microscopy (SEM).
