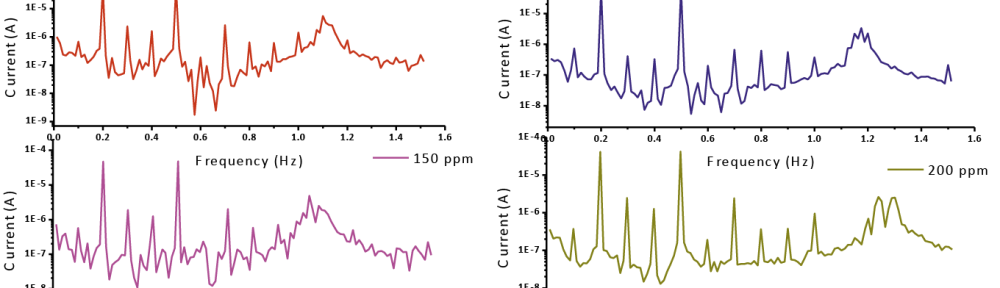Author Archives: Prof. Dr. A. S. Fouda
Delonix Regia Leaf Extract as Environmental Friendly and Safe Corrosion Inhibitor for Carbon Steel in Aqueous Solutions
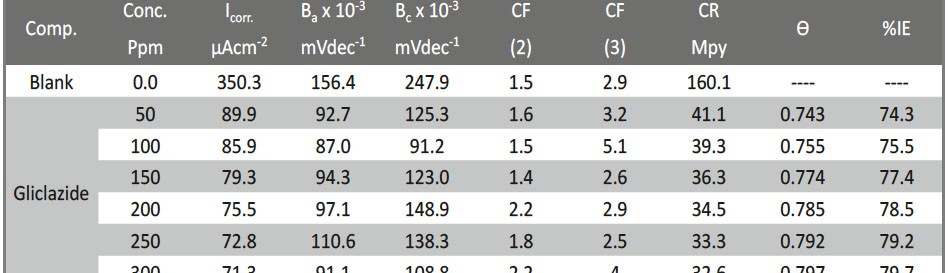
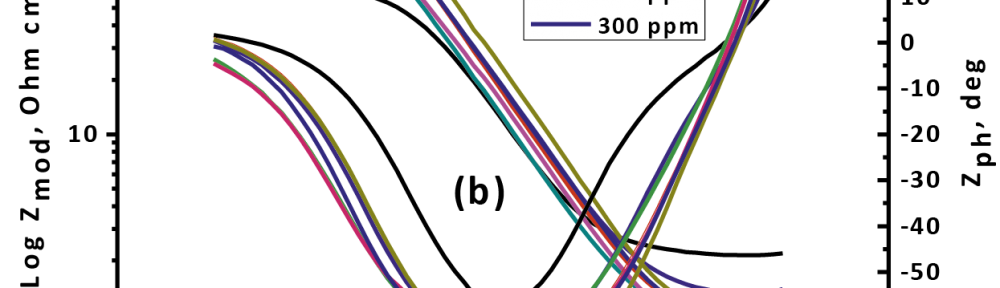
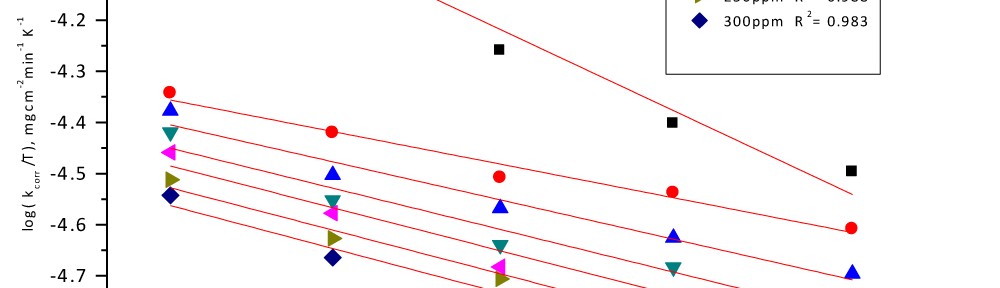
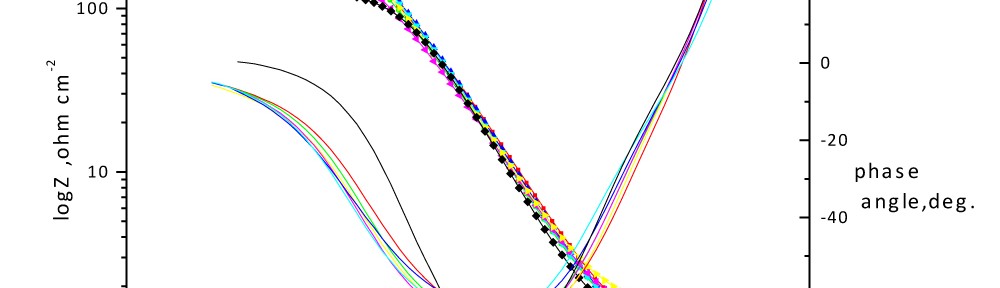
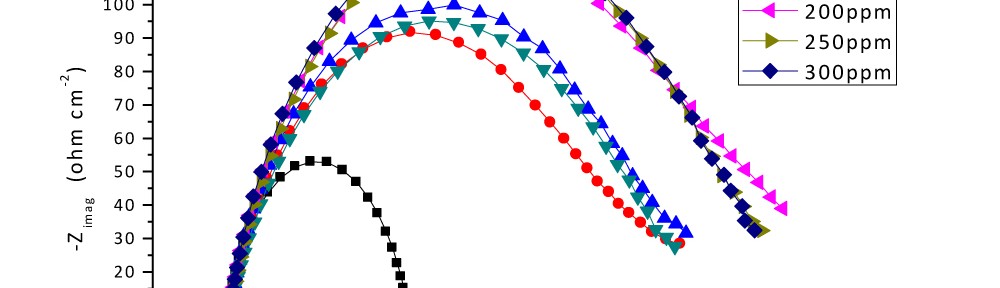
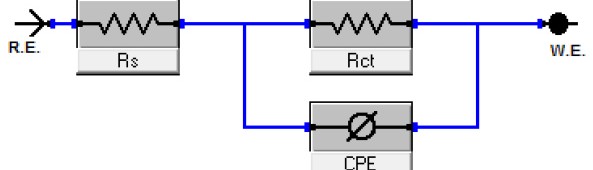
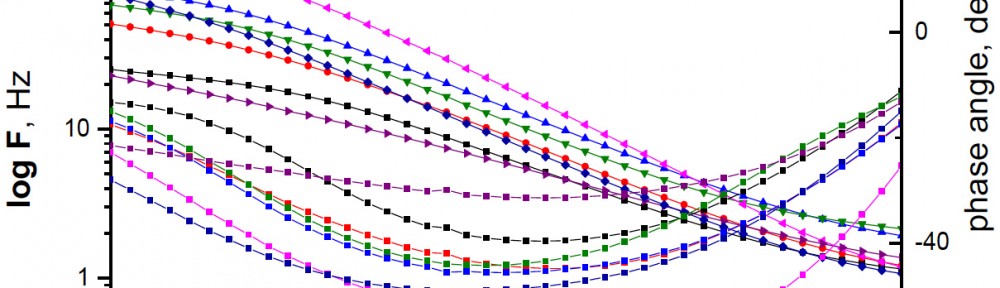
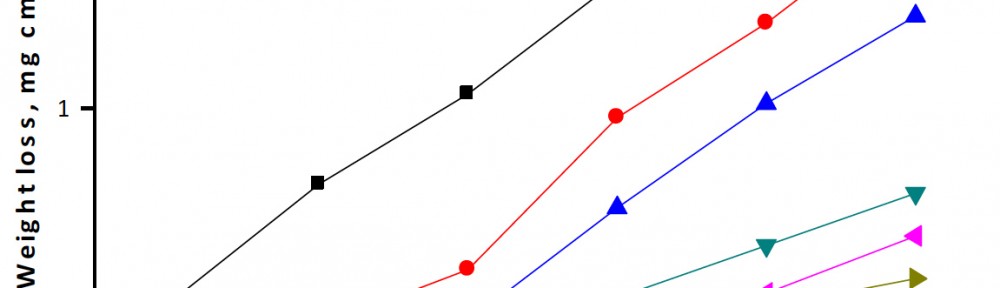
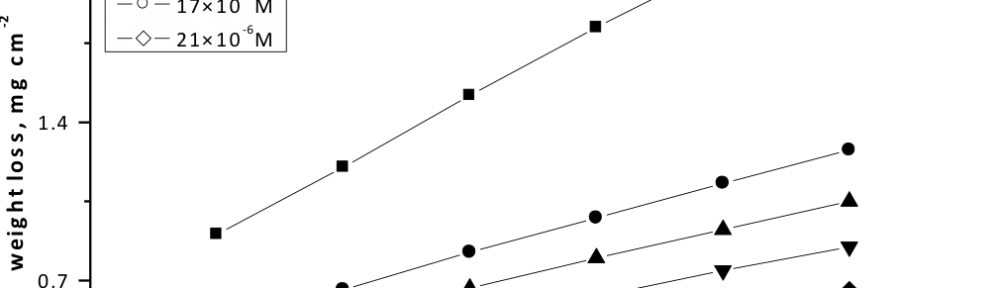
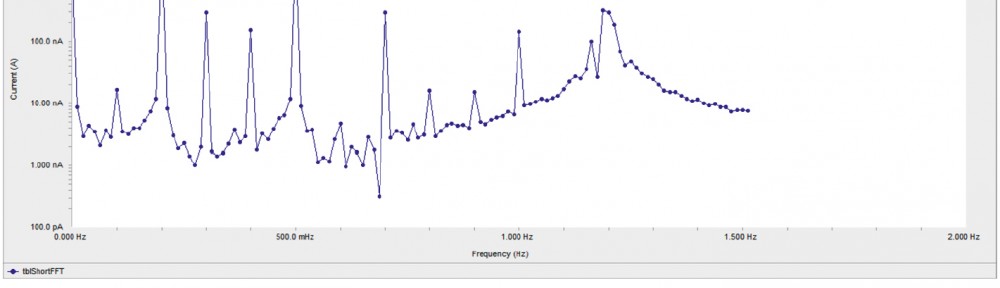
Delonix regia leaf extract activity as a green corrosion inhibitor (environmental friendly) for carbon steel (CS) in 1M HCl has been studied using weight loss (WL), potentiodynamic polarization (PP), electrochemical frequency modulation (EFM) and electrochemical impedance spectroscopy (EIS). The weight loss results show that Delonix regia leaf extract is an excellent corrosion inhibitor. The inhibition efficiency (IE) increases with temperature from 25 to 45oC, reaching a maximum value of 78.8 % at the highest concentration of 300 ppm at the temperature of 45oC. Polarization measurements demonstrate that the Delonix regia leaf extract acts as a mixed type inhibitor. Nyquist plot illustrates that on increasing Delonix regia leaf extract dose, the charge transfer increases and the double layer capacitance decreases. The adsorption of Delonix regia leaf extract on CS obeys Temkin adsorption isotherm.
Adsorption and inhibitive properties of aqueous extracts of rosmarinus as a green corrosion extract for copper in HNO3
The efficiency of plant extract as corrosion extract for copper in 1M HNO3 medium was carried out using weight loss, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. The results showed variation in inhibition performance of the extract with varying concentration, immersion time and temperature. Langmuir isotherm was tested to describe the adsorption behavior of the extract on the copper surface. Potentiodynamic polarization study clearly revealed that this extract acts as a mixed type inhibitor i.e. the addition of the extract enhances both cathodic and anodic reactions. The results of the electrochemical impedance study showed a decrease in double layer capacitance and an increase in the charge transfer resistance. The results showed that rosmarinus extract could play significant role as corrosion inhibitor for copper in 1M HMO3.
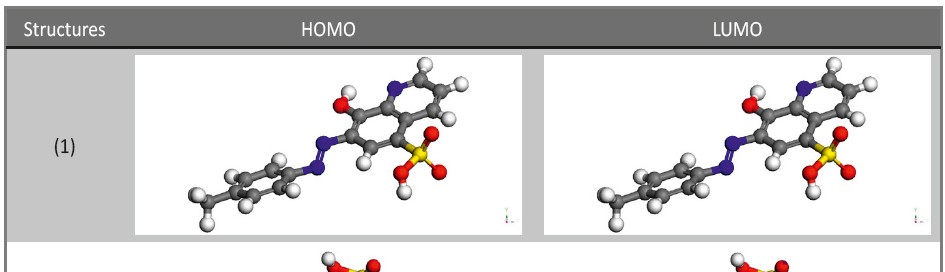
8-hydroxy-7-phenylazo-quinoline-5-sulfonicacid derivatives as corrosion inhibitors for copper in nitric acid solutions
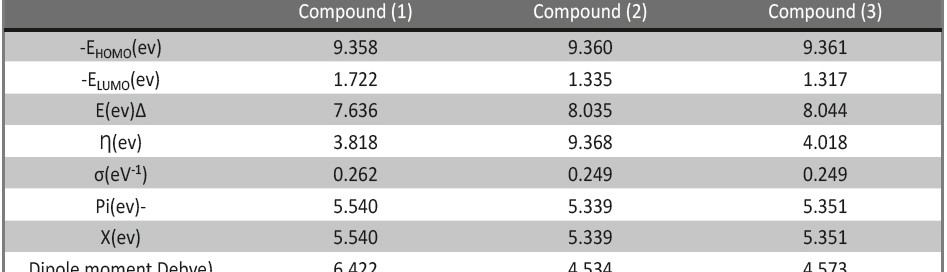
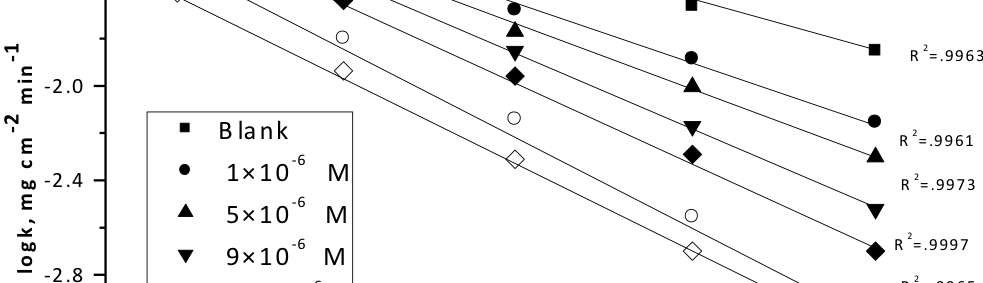
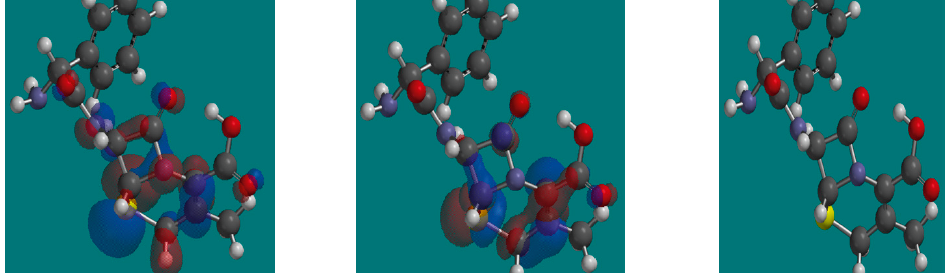
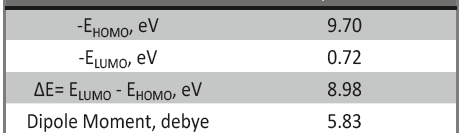
Corrosion Inhibition of copper in nitric acid by 8-hydroxy-7-phenylazo-quinoline-5-sulfonicacid derivatives have been studied using weight loss and electrochemical measurements. The results showed that these derivatives act as moderate corrosion inhibitor for copper at all concentrations of these derivatives. All results indicate that the inhibition efficiency increases with increasing inhibitor concentrations. Polarization curves revealed that these derivatives are mixed type inhibitors. The adsorption of these derivatives on the surface of the copper specimens obeys Temkin adsorption isotherm. Some thermodynamic and kinetic parameters for the corrosion process were calculated and discussed. Some quantum chemical parameters for these derivatives calculated by the density function theory (DFT) semi-empirical method to provide further insight into the mechanism of inhibition of the corrosion process.
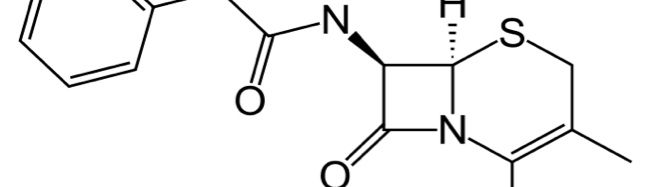
Cephalexin as Efficient Corrosion Inhibitor for Mild Steel in Acidic Media Chemical, Electrochemical and Thermodynamic Studies
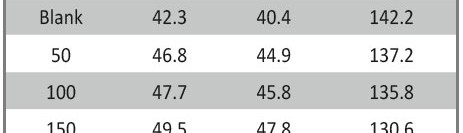
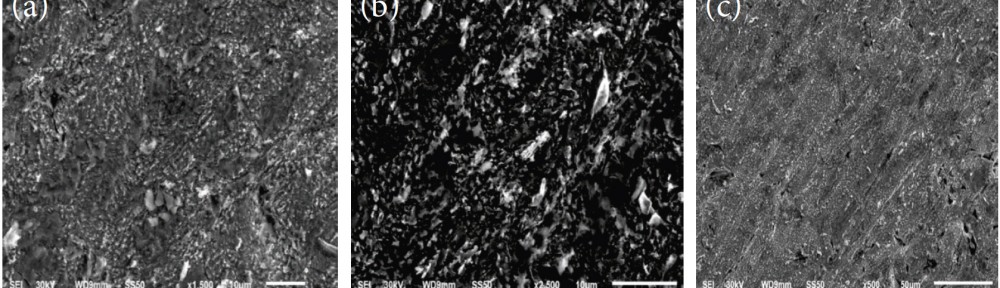
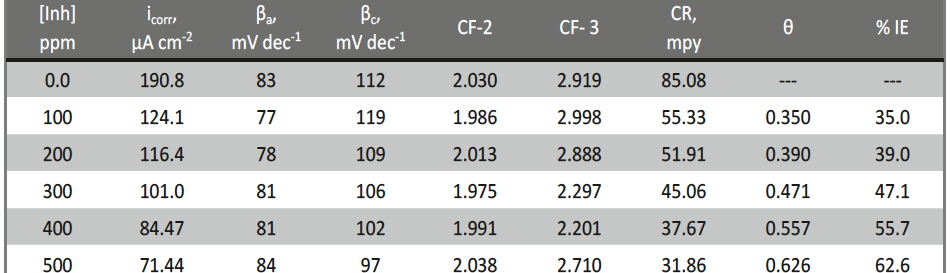
The corrosion inhibition of mild steel in 0.5 M H2SO4 solution by pharmaceutical antibacterial drug named Cephalexin has been investigated by using weight loss, potentiodynamic polarization, electrochemical frequency modulation technique (EFM) and electrochemical impedance spectroscopy (EIS) measurements. The polarization data showed that this drug is mixed-type inhibitor. The percentage inhibition efficiency was found to increase with increasing the concentration of the drug and with decreasing temperature. The Langmuir`s isotherm was found to provide an accurate description of adsorption behavior of this drug. Some thermodynamic parameters were computed and discussed. The correlations between advanced quantum chemical concepts and inhibition efficiency was found and discussed. The data obtained from different methods are in good agreement.