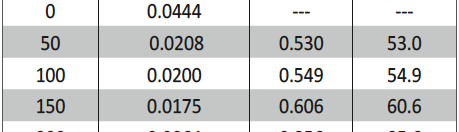
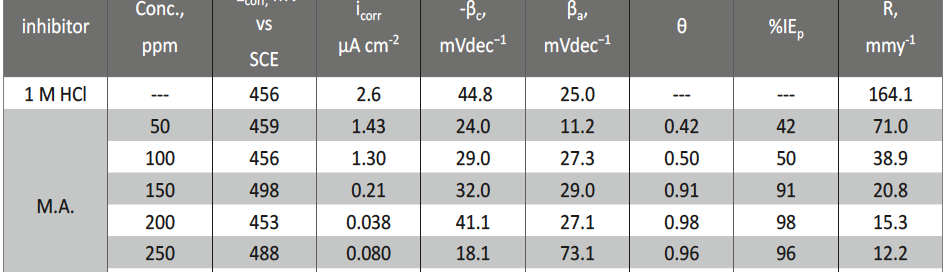
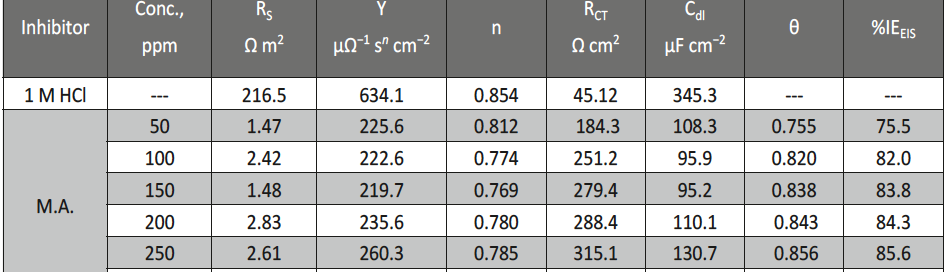
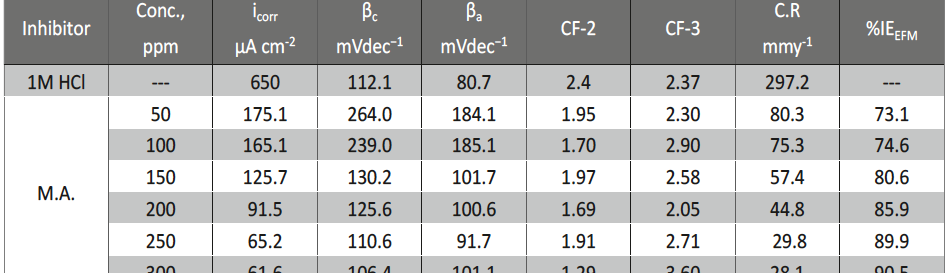
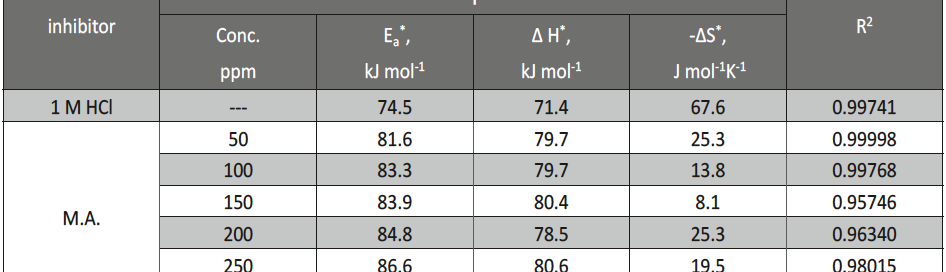
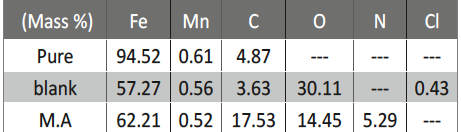
The protection effect of malonic acid on carbon steel corrosion was studied in aerated stagnant 1M HCl solutions at 250C. Measurements were conducted under different experimental conditions using weight loss, Tafel polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. malonic acid was found to be good inhibitor of carbon steel corrosion in1 M HCl. The adsorption of this inhibitor is found to obey the Langmuir adsorption isotherm. The calculated activation energies proposed that the inhibitor molecules being physically adsorbed onto the metal surface. Polarization data revealed that this compound behave as mixed type inhibitor.
Tag Archives: green inhibitor
Allium sativum (Garlic) as green corrosion inhibitor for low carbon steel in HCl solutions
The use of allium sativum (garlic) as a green inhibitor for the corrosion of low carbon steel in 1M HCl has been studied using potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), electrochemical frequency modulation (EFM) techniques and weight loss measurements. Allium sativum has been proved to be good inhibitor. This reduction in the corrosion rate was due to the formation of an external layer formed by S-containing film present in the extract which was adsorbed physically on the metal surface. Allium sativum acted as a mixed type of inhibitor. The inhibition efficiency increases with increasing the inhibitor concentration, but decreases with raising the temperature. The adsorption of allium sativum on the metal surface follows Temkin’s adsorption isotherm. From EFM the causality factors are very close to theoretical values which indicate that the measured data are of good quality. Nyquist plots show a single capacitive loop in uninhibited and inhibited solutions.