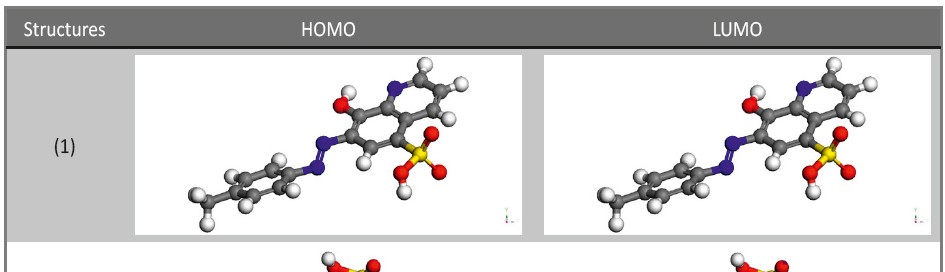
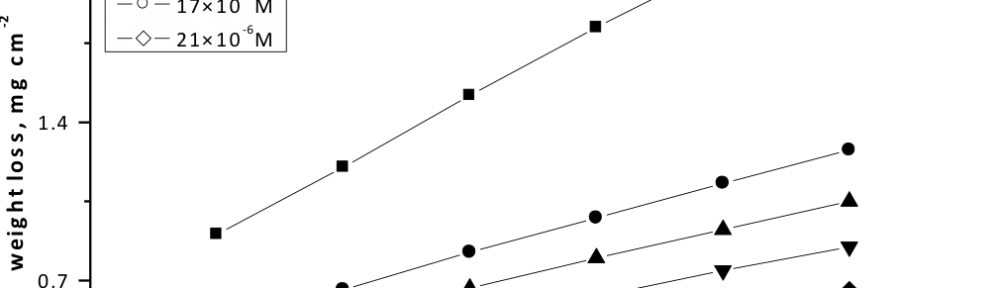
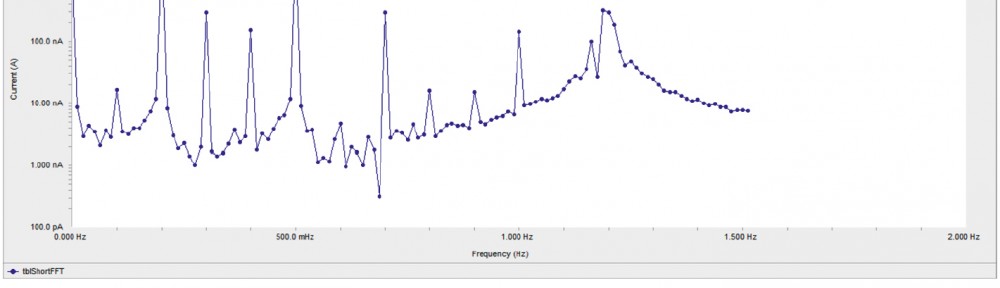
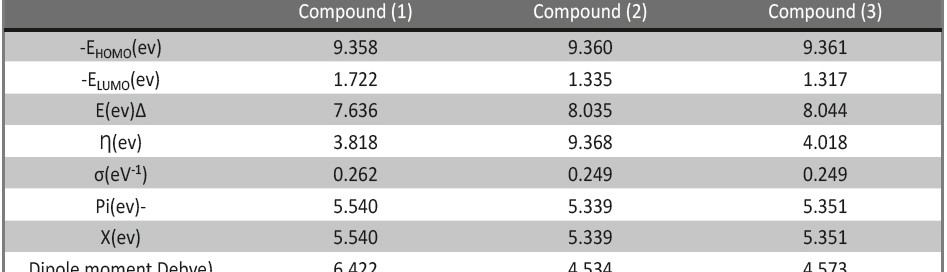
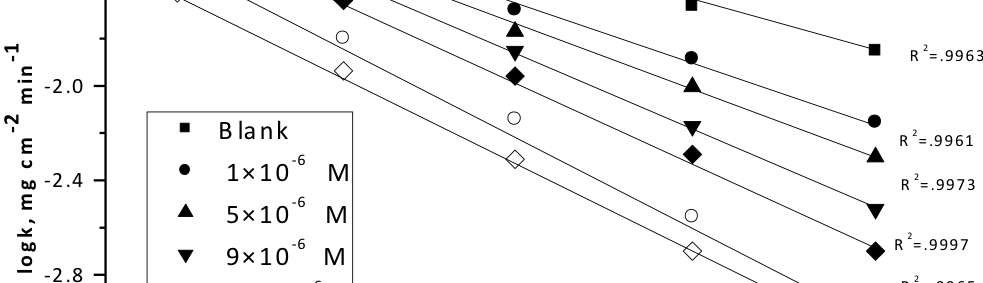
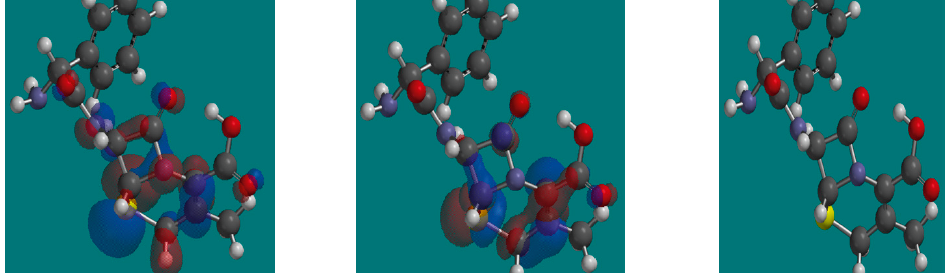
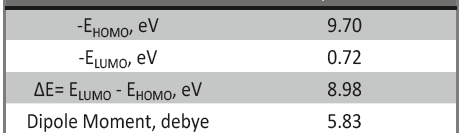
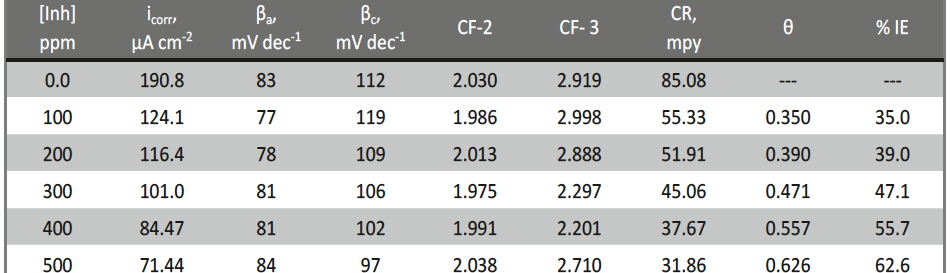
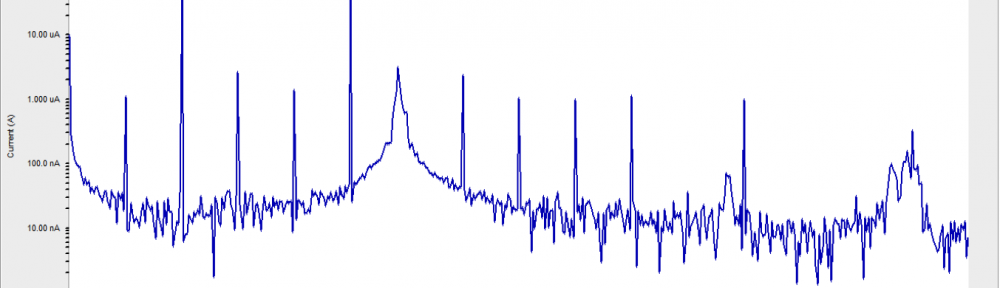
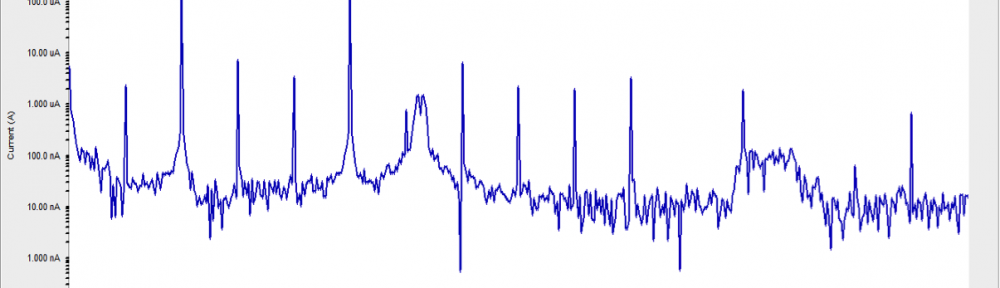
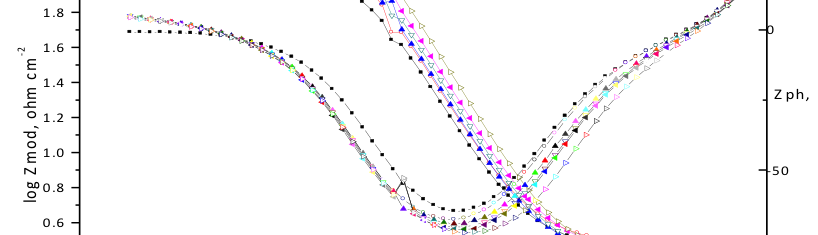
Corrosion Inhibition of copper in nitric acid by 8-hydroxy-7-phenylazo-quinoline-5-sulfonicacid derivatives have been studied using weight loss and electrochemical measurements. The results showed that these derivatives act as moderate corrosion inhibitor for copper at all concentrations of these derivatives. All results indicate that the inhibition efficiency increases with increasing inhibitor concentrations. Polarization curves revealed that these derivatives are mixed type inhibitors. The adsorption of these derivatives on the surface of the copper specimens obeys Temkin adsorption isotherm. Some thermodynamic and kinetic parameters for the corrosion process were calculated and discussed. Some quantum chemical parameters for these derivatives calculated by the density function theory (DFT) semi-empirical method to provide further insight into the mechanism of inhibition of the corrosion process.
Tag Archives: corrosion inhibition
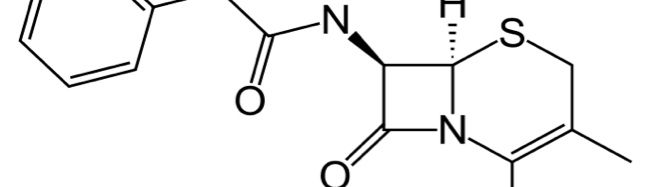
Cephalexin as Efficient Corrosion Inhibitor for Mild Steel in Acidic Media Chemical, Electrochemical and Thermodynamic Studies
The corrosion inhibition of mild steel in 0.5 M H2SO4 solution by pharmaceutical antibacterial drug named Cephalexin has been investigated by using weight loss, potentiodynamic polarization, electrochemical frequency modulation technique (EFM) and electrochemical impedance spectroscopy (EIS) measurements. The polarization data showed that this drug is mixed-type inhibitor. The percentage inhibition efficiency was found to increase with increasing the concentration of the drug and with decreasing temperature. The Langmuir`s isotherm was found to provide an accurate description of adsorption behavior of this drug. Some thermodynamic parameters were computed and discussed. The correlations between advanced quantum chemical concepts and inhibition efficiency was found and discussed. The data obtained from different methods are in good agreement.
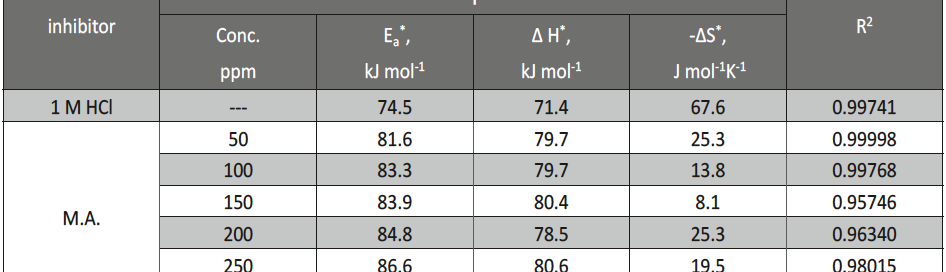
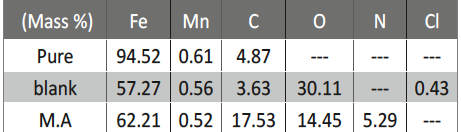
Malonic Acid as Corrosion Inhibitor for Carbon Steel in 1 M Hydrochloric Acid Solutions
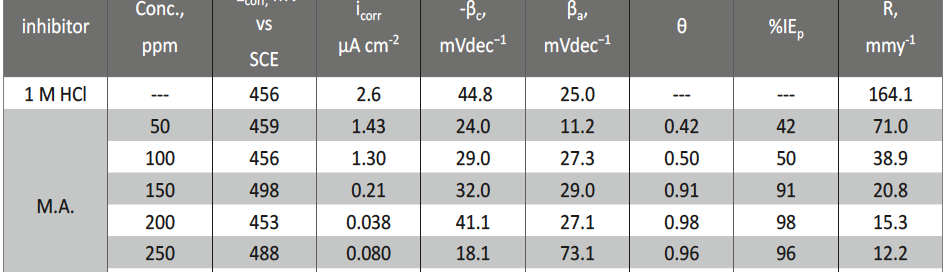
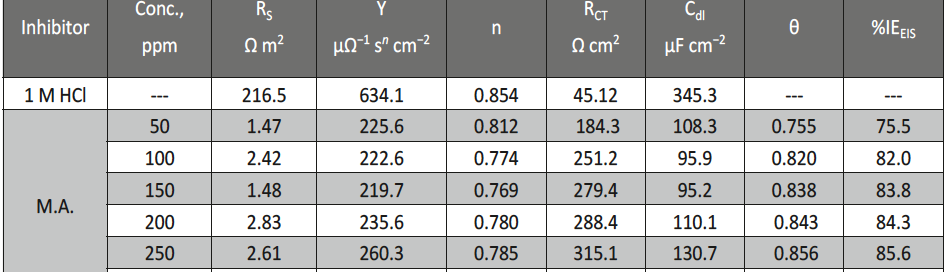
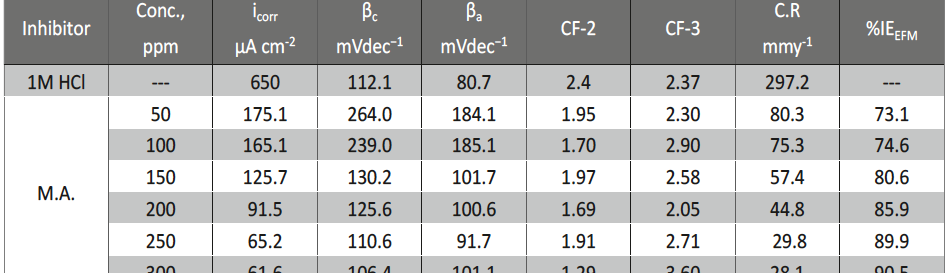
The protection effect of malonic acid on carbon steel corrosion was studied in aerated stagnant 1M HCl solutions at 250C. Measurements were conducted under different experimental conditions using weight loss, Tafel polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. malonic acid was found to be good inhibitor of carbon steel corrosion in1 M HCl. The adsorption of this inhibitor is found to obey the Langmuir adsorption isotherm. The calculated activation energies proposed that the inhibitor molecules being physically adsorbed onto the metal surface. Polarization data revealed that this compound behave as mixed type inhibitor.
Adsorption and Corrosion Inhibition Characteristics of Some Thiophene-3-Carbohydrazide Derivatives on Low Carbon Steel in Hydrochloric Acid Solutions
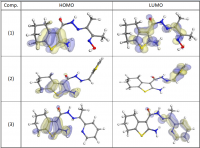
New compounds of corrosion inhibitors namely amino-N’-(3-(hydroxyimino)butan-2-ylidene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (1), amino-N’-(thiophen-2-ylmethylene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (2) and amino-N’-(1-(pyridin-2-yl)ethylidene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (3) were synthesized and its inhibiting action on the corrosion of carbon steel in 1 M hydrochloric acid at 25ºC was investigated by various corrosion monitoring techniques. A Potentiodynamic polarization, AC impedance and electrochemical frequency modulation methods have been used. Potentiodynamic polarization studies showed that these derivatives were mixed type inhibitors. The effect of temperature on the corrosion behavior of carbon steel in 1 M HCl with the addition of these compounds were studied in the temperatures 25 and 45ºC. The adsorption of these inhibitors on carbon steel surface from hydrochloric acid obeyed the Langmuir adsorption isotherm. Quantum chemical method is used to explore the relationship between the inhibitors molecular properties and their inhibition efficiency.
The Use of Molybdate, Chromate and Tungstate Anions as Corrosion Inhibitors for Steel in Sulfide Polluted Salt Water
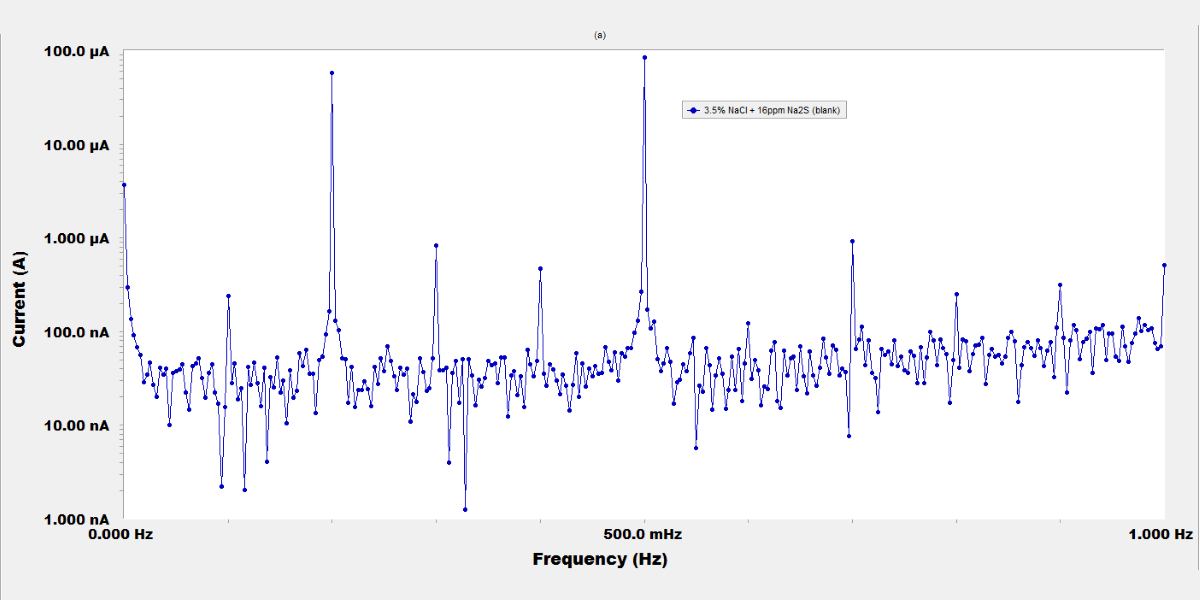
The inhibiting effect of molybdate, chromate, and tungstate salts on the corrosion of steel used in sanitation plants was investigated by potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), and electrochemical frequency modulation (EFM) techniques. The sulfide polluted salt water was simulated by a 3.5 % NaCl and 16 ppm Na2S solution. The results revealed that these inorganic anions are very good inhibitors. Potentiodynamic polarization curves indicated an anodic-type inhibition. The increased concentration of inorganic compounds increases the inhibition; at 250 ppm concentration the inhibition efficiency reached to 95 %, 91.6 %, and 87.5 % for MoO42-, CrO42-, and WO42-, respectively. The adsorption of the inhibitors on the metal surface basically obeys the Langmuir adsorption isotherm equation. Results of EIS measurements suggested that the dissolution of the steel occurs under activation control, and a passive film is probably formed on the metal surface. The electrochemical kinetic parameters calculated from EFM spectra confirm the polarization data.