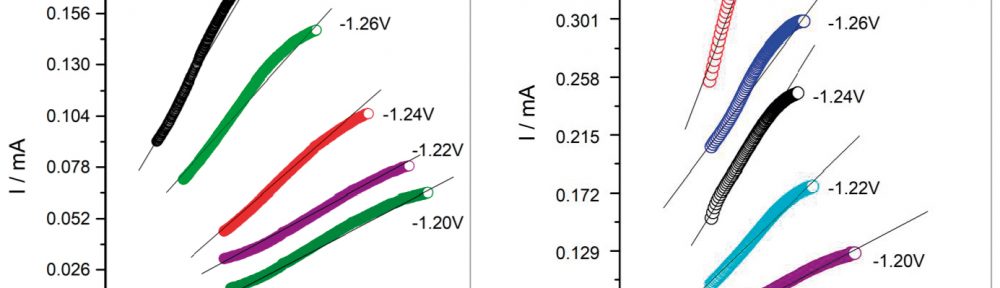
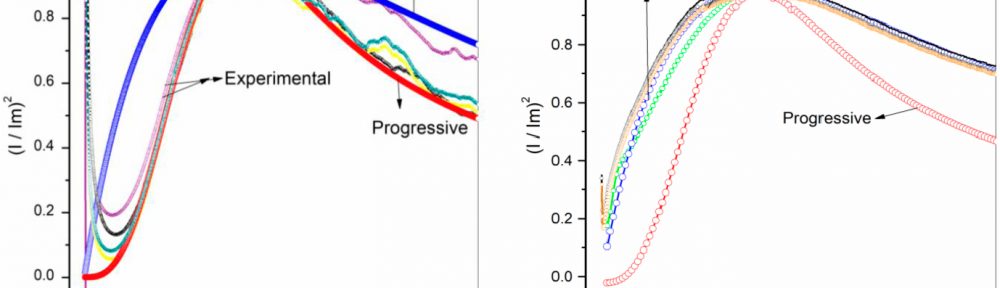
In present investigation, a new brightener was synthesized by condensation of 3, 4, 5-Trimethoxy benzaldehyde and Glycine (TG). Hull cell experiments were conducted to optimize the plating bath components and operating parameters. To examine the influence of TG on nucleation mechanism of Zn-Co alloy electrodeposition, cyclic voltammetry and chronoamperometry study was carried out. Schariffker and Hills model was used to analyze current transients, which in presence of TG confirmed instantaneous nucleation. Corrosion studies were done using potentiodynamic polarization and electrochemical impedance spectroscopic technique, in 3.5 wt. % NaCl for bright and dull zinc-cobalt alloy coatings. Phase structure, surface morphology and brightness of the deposit were characterized by X-ray diffraction analysis, scanning electron microscopy and reflectance studies. These studies revealed the role of TG in modifying the nucleation mechanism and surface morphology of zinc-cobalt alloy crystallites and thereby producing a bright corrosion resistant Zn-Co alloy coating on mild steel substrate.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany