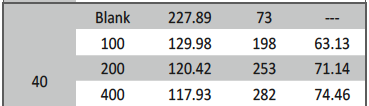
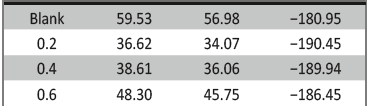
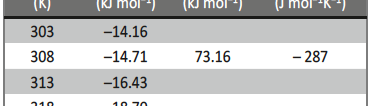
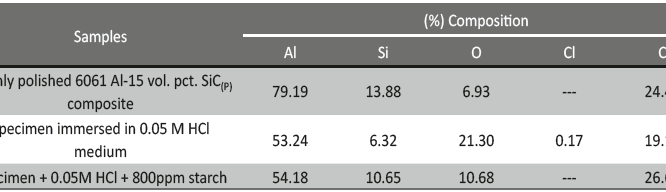
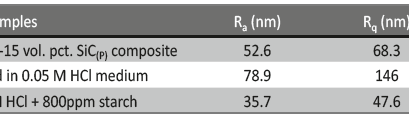
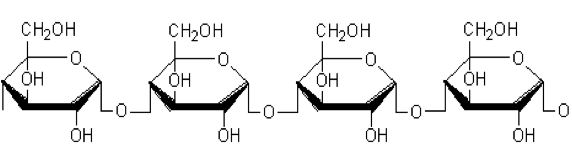
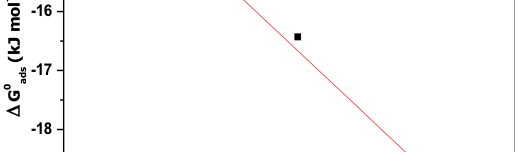
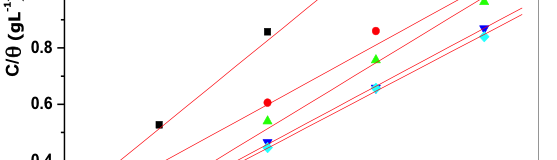
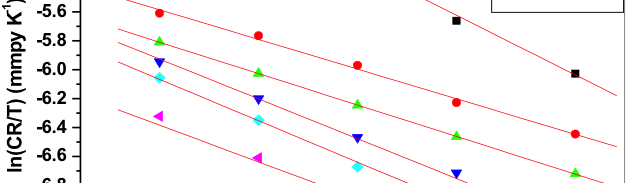
The influence of biopolymer starch as corrosion inhibitor on 6061 Al-15 vol. pct. SiC(p) composite in 0.05M hydrochloric acid was studied by potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) technique. The surface morphology was studied using SEM, EDX, AFM and XRD techniques. The results showed that the inhibition efficiency of starch increased with increasing inhibitor concentrations and also with increase in temperatures. Starch acted as a mixed inhibitor and underwent chemical adsorption following Langmuir adsorption isotherm.
Tag Archives: SEM
Electrochemical Investigation on the Corrosion Behavior of Combined Addition of Cu and Ni to Al-Si-Mg Alloy in 0.1M NaCl Solution

The purpose of this paper is to understand the effect of 2Cu+2Ni addition on electrochemical corrosion behavior of thermal treated Al-6Si-0.5Mg alloy in 0.1M NaCl solution. The corrosion of the thermal treated samples was characterized by electrochemical potentiodynamic polarization technique consisting of linear polarization method using the fit Tafel plot and electrochemical impedance spectroscopy (EIS) techniques. Generally, from the linear polarization, the corrosion rate decreases at thermal treated Al-6Si-0.5Mg-2Cu-2Ni alloy (Alloy-2). The corrosion behavior of the Alloy-2 in the 0.1M NaCl solution showed better resistance than the Alloy-1. The EIS test results also showed that the changing of charge transfer resistance (Rct) is significant with the combined addition of 2Cu+2Ni to Al-6Si-0.5Mg alloy. The magnitude of the noble shift in the open circuit potential (OCP), corrosion potential (Ecorr) and pitting corrosion potential (Epit) increased with the addition of 2Cu+2Ni to Al-6Si-0.5Mg alloy.
Magnetic property and corrosion resistance of electrodeposited nanocrystalline cobalt-nickel alloys
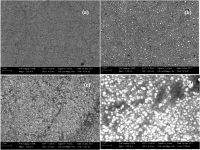
In the present investigation we have galvanostatically synthesized Co-Ni nanocrystalline alloys on copper substrate. The effect of current density (c.d.) on composition, surface morphology and phase structure were studied for explaining the magnetic and corrosion resistance of the alloy. The bath found to exhibit the preferential deposition of less noble Co than Ni, and at no conditions of c.d., the deposition has changed from anomalous to normal type. Surface morphology and structural characteristics of the deposits were examined using scanning electron microscopy (SEM) and X-ray diffraction (XRD) analysis. As composition of the alloy varied, consequent to the c.d. a change of hexagonal close packing structure (hcp) to face centered cubic structure (fcc) was observed. Finally, the conditions responsible for peak magnetic property and corrosion resistance were optimized. Factors responsible for improved functional properties were explained in terms of surface morphology and crystalline grain size of the coatings.
Effect of additives and operating parameters on deposit characters of Ni-Cd alloy
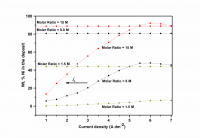
The Ni-Cd alloy coating was electrodeposited on mild steel (MS) from acid chloride bath using gelatin and glycerol as additives, individually and in combination. The bath composition and operating parameters have been optimized by conventional Hull cell method. The effect of current density (c.d.) on Ni content of the alloy was studied at different molar ratio of metal ions in the bath. The effects of c.d. and temperature on thickness, hardness, and composition and corrosion rate (CR) of the coatings were studied. Cyclic voltammetry (CV) study showed that (gelatin + glycerol) has significant effect on process of deposition and (gelatin + glycerol) worked synergistically to increase the Ni content by their preferential deposition and by suppressing the deposition of more readily depositable Cd2+ ions. Ni-Cd bath having both [Ni2+]/[Cd2+] = 1.5 and 8.0 exhibited anomalous type of codeposition at all c.d.’s studied. Corrosion behavior of the coatings evaluated by electrochemical methods demonstrated that the coating from bath [Ni2+]/[Cd2+] = 15, deposited at 4.0 A dm-2 is the most corrosion resistant. The superior corrosion resistance of Ni-Cd coatings at optimal c.d. was attributed to specific Ni (111), Ni (200), Cd (200) and Ni-Cd (862) reflections, evidenced by XRD study. The surface morphology was analyzed using SEM study, and results are discussed.
Electrochemical Investigation on the Corrosion Behaviour of Mg-Al-Zn-Mn (GA9) Alloy in Sodium Chloride Medium
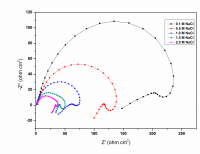
The corrosion behavior of Mg-Al-Zn-Mn (GA9) alloy in sodium chloride solutions was studied over a range of concentrations and solution temperatures by electrochemical techniques like potentiodynamic polarization (PP) and electrochemical impedance spectroscopy (EIS). The studies were carried out in solutions with NaCl of concentrations between 0.1M – 2M; and at different temperatures in the range of 30 ᴼC – 50 ᴼC. The studies have revealed that the corrosion rate of GA9 magnesium alloy increases with the increase in temperature and also with the increase of NaCl concentration in the medium. Activation parameters like activation energy, enthalpy of activation and entropy of activation for evaluation of the corrosion process were calculated. The results from both the techniques are in good agreement with each other. The alloy surface morphology was studied before and after corrosion using scanning electron microscopy (SEM).

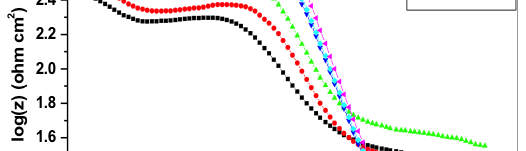
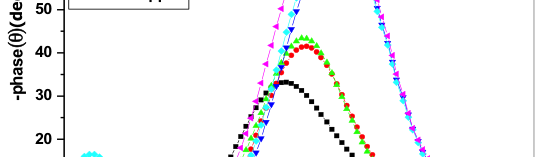
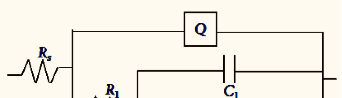
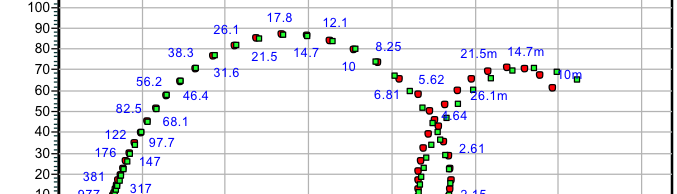
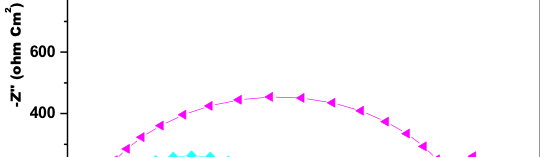
![Fig. 2: Potentiodynamic polarization curves for the corrosion of 6061 Al-15 vol. pct. SiC(P) composite in 0.05M HCl containing various [starch] at 35 °C](https://www.jept.de/wp-content/uploads/2016/02/pic2-578x166.png)







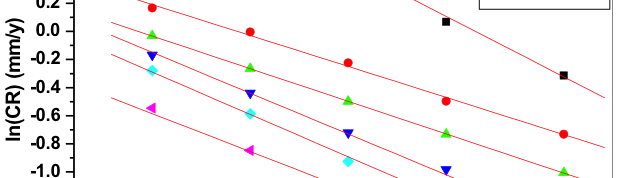
![Tab. 2: Results of Potentiodynamic polarization studies for corrosion of 6061 Al-15 vol. pct. SiC(P) composite in 0.05M HCl containing various [starch]](https://www.jept.de/wp-content/uploads/2016/02/tab2-756x217.png)