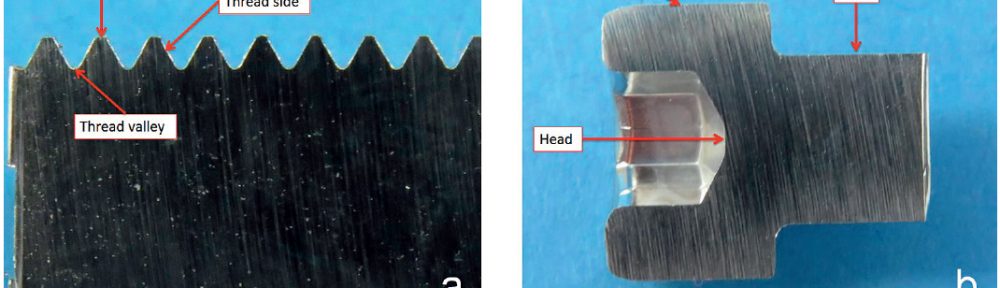
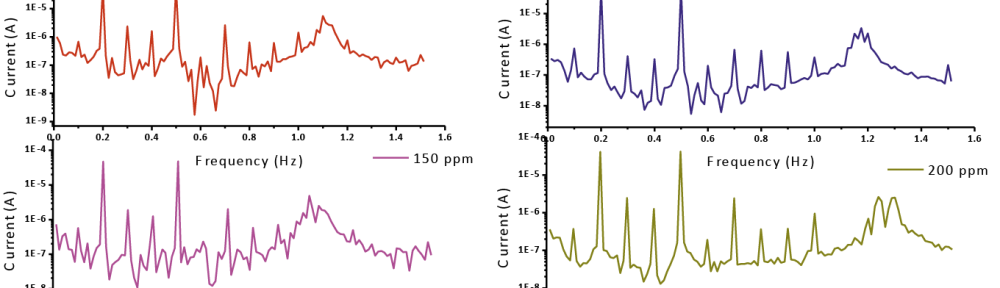New generation of acid Zn-Ni electrolyte for barrel application (Part 2)
The demand for Zinc Nickel coatings continuously increases in the automotive industry. Especially interesting are zinc nickel alloys with a nickel incorporation of 12–16 %, due to their high corrosion protection as well as superior wear and heat resistance as compared to pure zinc and other zinc alloy coatings.
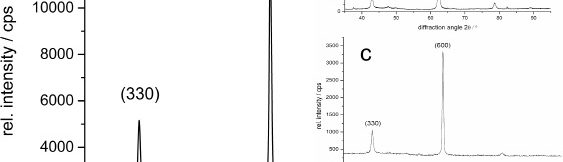
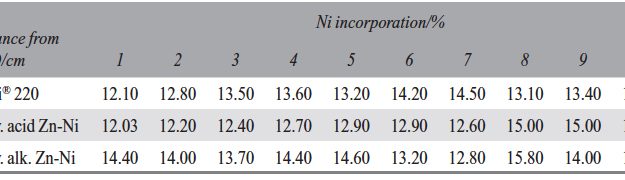
Despite many advantages of acid Zn-Ni electrolytes there are still some areas of application, like barrel plating or plating of complex-shaped parts, believed to be reserved for alkaline processes. In this paper zinc nickel coatings deposited from ammonium and boric acid-free acid zinc nickel electrolytes, with improved throwing power for rack and barrel applications are investigated. Their corrosion resistance, ductility and hardness will be presented. Moreover, their texture and morphology will be investigated using SEM, XRD and FIB methods. In the end thickness distribution and Ni-incorporation will be presented and compared to alkaline systems.
New generation of acid Zn-Ni electrolyte for barrel application (Part 1)
The demand for Zinc Nickel coatings continuously increases in the automotive industry. Especially interesting are zinc nickel alloys with a nickel incorporation of 12–16 %, due to their high corrosion protection as well as superior wear and heat resistance as compared to pure zinc and other zinc alloy coatings. Despite many advantages of acid Zn-Ni electrolytes there are still some areas of application, like barrel plating or plating of complex-shaped parts, believed to be reserved for alkaline processes. In this paper zinc nickel coatings deposited from ammonium and boric acid-free acid zinc nickel electrolytes, with improved throwing power for rack and barrel applications are investigated. Their corrosion resistance, ductility and hardness will be presented. Moreover, their texture and morphology will be investigated using SEM, XRD and FIB methods. In the end thickness distribution and Ni-incorporation will be presented and compared to alkaline systems.
Delonix Regia Leaf Extract as Environmental Friendly and Safe Corrosion Inhibitor for Carbon Steel in Aqueous Solutions
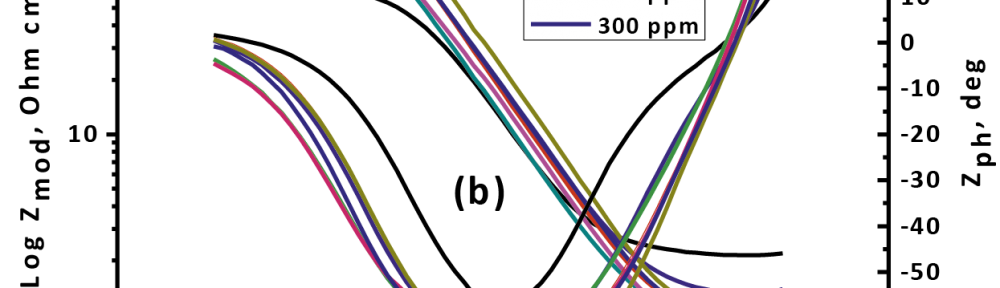
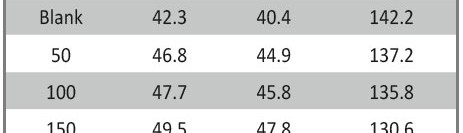
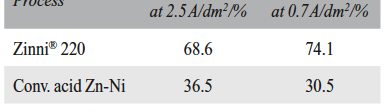
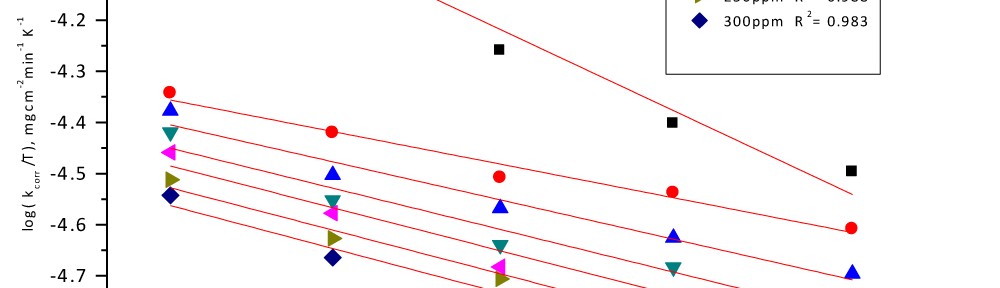
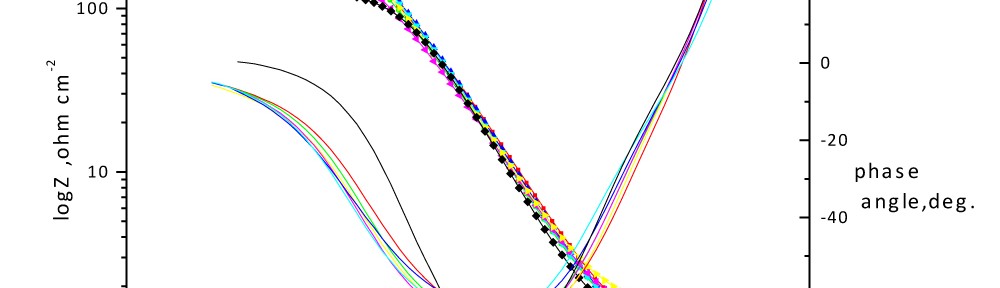
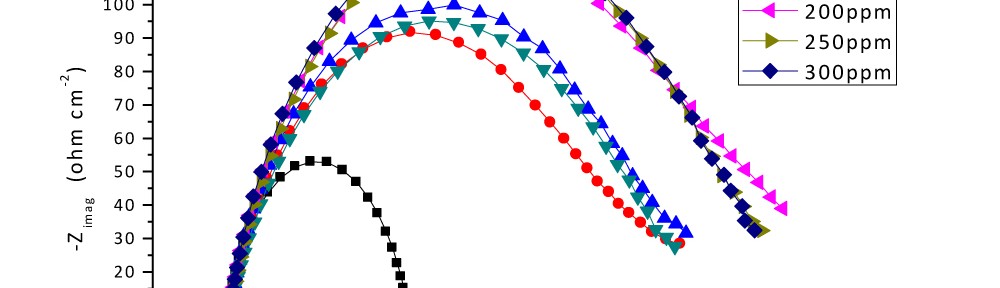
Delonix regia leaf extract activity as a green corrosion inhibitor (environmental friendly) for carbon steel (CS) in 1M HCl has been studied using weight loss (WL), potentiodynamic polarization (PP), electrochemical frequency modulation (EFM) and electrochemical impedance spectroscopy (EIS). The weight loss results show that Delonix regia leaf extract is an excellent corrosion inhibitor. The inhibition efficiency (IE) increases with temperature from 25 to 45oC, reaching a maximum value of 78.8 % at the highest concentration of 300 ppm at the temperature of 45oC. Polarization measurements demonstrate that the Delonix regia leaf extract acts as a mixed type inhibitor. Nyquist plot illustrates that on increasing Delonix regia leaf extract dose, the charge transfer increases and the double layer capacitance decreases. The adsorption of Delonix regia leaf extract on CS obeys Temkin adsorption isotherm.
Influence of Alloy Composition on Performance of Zinc-Nickel Coatings
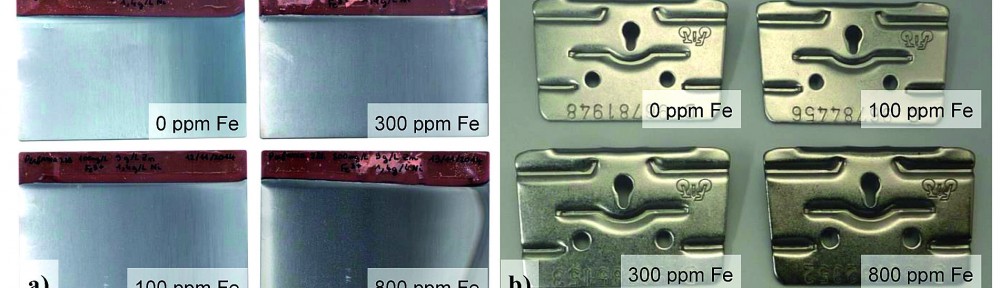
Electrodeposited zinc-nickel coatings are broadly used as sacrificial coatings for steel since many years in the automotive industry and for other high corrosion resistant applications. The best corrosion resistance is obtained with ZnNi deposits having 12–15 % Ni in the alloy. Many studies were performed showing the influence of nickel content in the alloy [1]. In industrial plating electrolytes other metals than Zn and Ni can be present. In alkaline zinc-nickel electrolytes mild steel is usually used as anode material. Depending on electrolyte composition and plating conditions more or less iron can be dissolved by anodic dissolution into the electrolyte. It is well known that the iron is codeposited into the zinc nickel alloy, but the effect on the alloy properties was never systematically investigated. In this study the influence of up to 800 mg/L iron in commercially used alkaline zinc nickel processes is investigated. Up to 8 % iron is amorphously codeposited in the alloy. No new iron containing phases could be detected by X-ray diffraction (XRD). ZnNi g-phases (Ni2Zn11/Ni5Zn21) are still the dominant phases, but plain orientation can be affected by iron codeposition. Corrosion properties are investigated by electrochemical measurements and neutral salt spray test. Whereas no huge difference in the corrosion properties between the bare ZnNi and ZnNiFe coatings was observed, the corrosion resistance with a subsequent trivalent chromium passivate can be drastically improved using iron in the alloy.