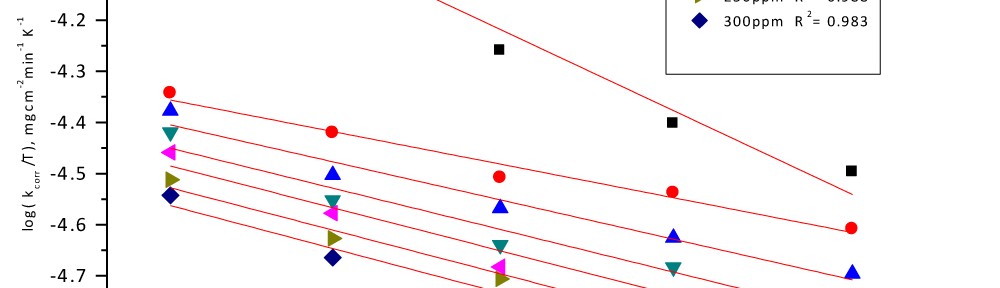
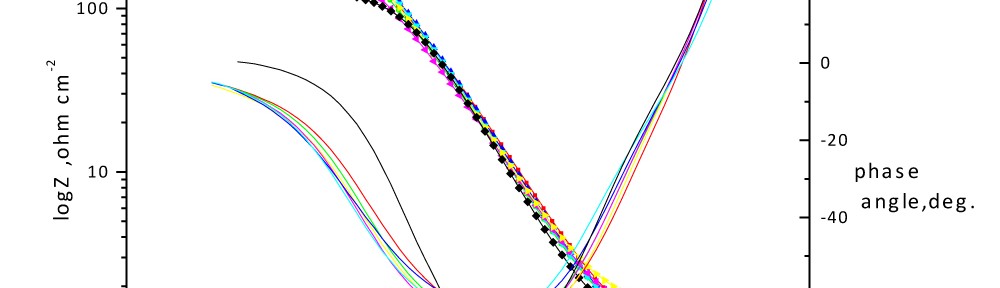
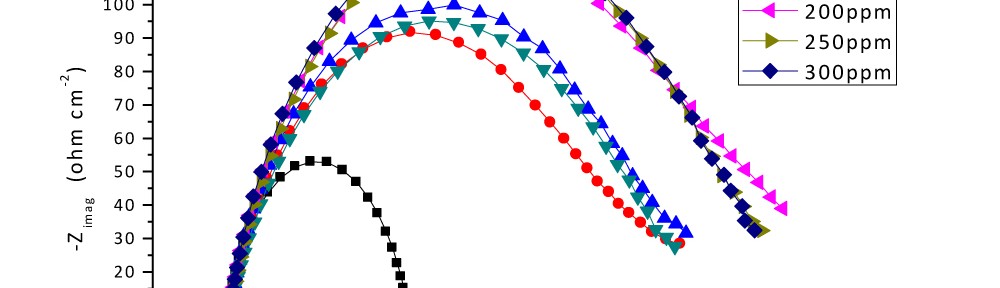
Delonix regia leaf extract activity as a green corrosion inhibitor (environmental friendly) for carbon steel (CS) in 1M HCl has been studied using weight loss (WL), potentiodynamic polarization (PP), electrochemical frequency modulation (EFM) and electrochemical impedance spectroscopy (EIS). The weight loss results show that Delonix regia leaf extract is an excellent corrosion inhibitor. The inhibition efficiency (IE) increases with temperature from 25 to 45oC, reaching a maximum value of 78.8 % at the highest concentration of 300 ppm at the temperature of 45oC. Polarization measurements demonstrate that the Delonix regia leaf extract acts as a mixed type inhibitor. Nyquist plot illustrates that on increasing Delonix regia leaf extract dose, the charge transfer increases and the double layer capacitance decreases. The adsorption of Delonix regia leaf extract on CS obeys Temkin adsorption isotherm.
Delonix Regia Leaf Extract as Environmental Friendly and Safe Corrosion Inhibitor for Carbon Steel in Aqueous Solutions
Delonix regia leaf extract activity as a green corrosion inhibitor (environmental friendly) for carbon steel (CS) in 1M HCl has been studied using weight loss (WL), potentiodynamic polarization (PP), electrochemical frequency modulation (EFM) and electrochemical impedance spectroscopy (EIS). The weight loss results show that Delonix regia leaf extract is an excellent corrosion inhibitor. The inhibition efficiency (IE) increases with temperature from 25 to 45oC, reaching a maximum value of 78.8 % at the highest concentration of 300 ppm at the temperature of 45oC. Polarization measurements demonstrate that the Delonix regia leaf extract acts as a mixed type inhibitor. Nyquist plot illustrates that on increasing Delonix regia leaf extract dose, the charge transfer increases and the double layer capacitance decreases. The adsorption of Delonix regia leaf extract on CS obeys Temkin adsorption isotherm.