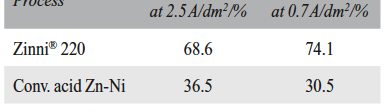
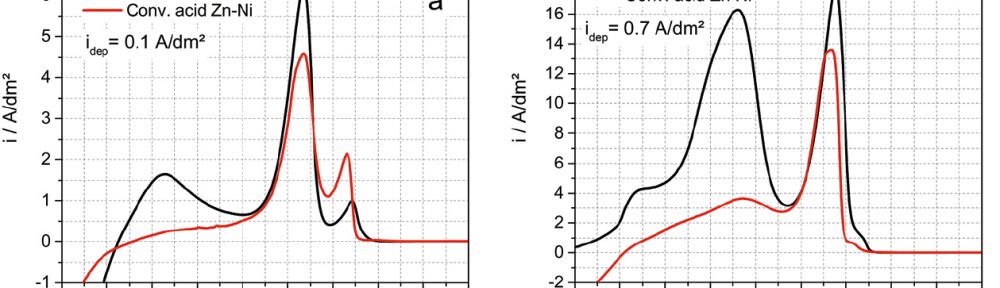
The demand for Zinc Nickel coatings continuously increases in the automotive industry. Especially interesting are zinc nickel alloys with a nickel incorporation of 12–16 %, due to their high corrosion protection as well as superior wear and heat resistance as compared to pure zinc and other zinc alloy coatings.
Despite many advantages of acid Zn-Ni electrolytes there are still some areas of application, like barrel plating or plating of complex-shaped parts, believed to be reserved for alkaline processes. In this paper zinc nickel coatings deposited from ammonium and boric acid-free acid zinc nickel electrolytes, with improved throwing power for rack and barrel applications are investigated. Their corrosion resistance, ductility and hardness will be presented. Moreover, their texture and morphology will be investigated using SEM, XRD and FIB methods. In the end thickness distribution and Ni-incorporation will be presented and compared to alkaline systems.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany