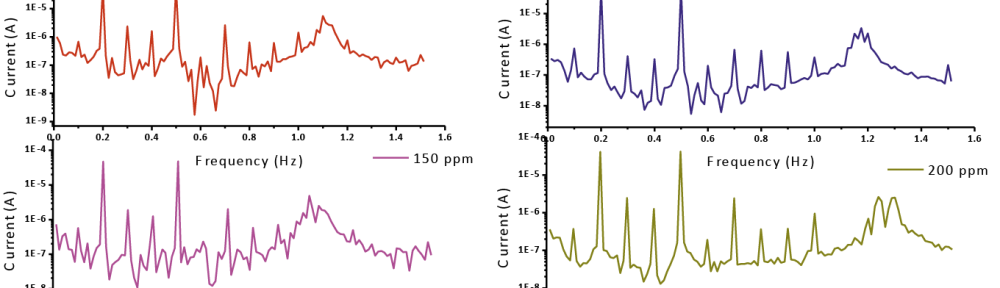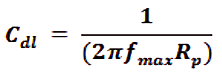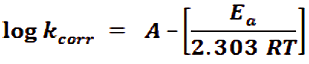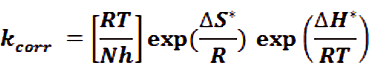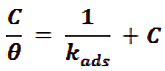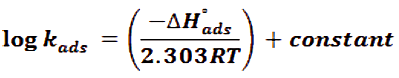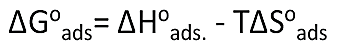1. Introduction
Corrosion is a major process that assumes an important role in the economy, safe and especially for metals [1]. The usage of medications is a standout amongst the most strategies for safety versus to erosion particularly in acidic medium [2]. Most well-known acid drugs are organic compounds containing nitrogen (N-heterocyclic)‚ sulfur‚ long carbon chain or aromatic and oxygen atoms. Among them‚ drugs have many advantages such as: high molecular size, highly soluble in water, availability, cheap‚ low toxicity‚ easy for using and easy production [3-5]. Natural heterocyclic mixes have been utilized for the erosion hindrance of CS [6-11], copper [12], aluminum [13-15], and various metals [16] in various watery medium. Adsorption of the drug molecules on the metal surface facilitates its inhibition [17]. A few medications have been discovered to be great corrosion inhibitors for metals such as: Biopolymer gave 86% IE for Cu in NaCl [18], pyromellitic diimide linked to oxadiazole cycle gave 84.6% IE for CS in HCl [19], 2-mercaptobenzimidazole gave 82% IE for CS in HCl [20], Antidiabetic Drug Janumet gave 88.7% IE for MS in HCl [21], Januvia gave 79.5% IE for Zn in HCl[22], Cefuroxime Axetil gave 89.9% IE for Al in HCl [23], Phenytoin sodium gave 79% for CS in HCl [24], Aspirin gave71% IE for MS in H2SO4 [25], Septazole gave 84.8% IE for Cu in HCl [26] and Chloroquine diphosphate gave 80% IE for MS in HCl [27].
Numerous authors for the most part concur that medications are drugs that can compete favorably with green inhibition of corrosion and that most medications can be synthesis from natural products. The select of some medication for drug of corrosion is taking in the following: 1) drug molecules contain oxygen, sulphur and nitrogen as active sites, 2) it is reportedly environmentally friendly furthermore vital in organic responses and 3) drugs can be easily produced and purified [28]. In recently years the drug’s uses as corrosion drugs for various metals result to their nontoxic nature [29]. The investigation of the relations between the adsorption and consumption hindrance is of awesome important. Heterocyclic mixes have demonstrated more hindrance effectiveness for CS in both HCl [30] and H2SO4 [31] arrangements.
2. Experimental detail
2.1. Carbon steel sample (CS)
The composition of CS sample is recorded in the (Tab. 1):
2.2. Chemicals
2.2.1. Drug
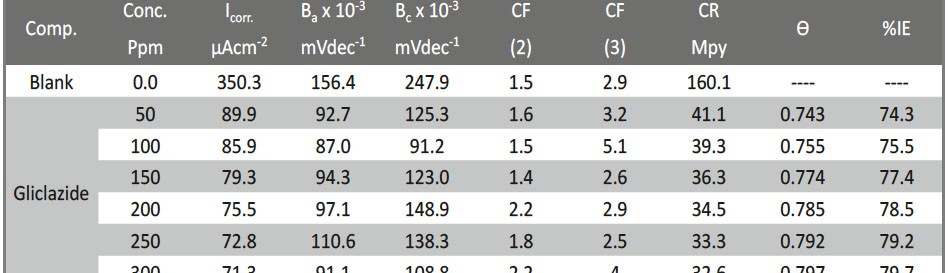
Gliclazide drug is used as an inhibitor which describing in (Tab. 2). It has been investigated purchased from Sandozinc and Pfizer inc. companies.
2.2.2. Solutions
The forceful arrangements, 1M HCl was set up by weakening of logical review (%37) HCl with distil water. The measurements scope of doses of the medication which utilized in the vicinity of or between (50 and 300 ppm).
2.3. Corrosion techniques
2.3.1. The (WL) technique
Collections data of the (WL) technique were taken by utilizing square coins samples. The area of surface is (2 cm x 2 cm) x 2 which exposed to the corrosive medium that used. The specimen coin polisher by SiC papers for different sizes (400, 600, 800, 1000 and 1200), clean with acetone, then clean with bi-distilled water and finally dried by filter paper. The (WL) data were achieves in a 100 ml glass beaker which put it in a thermostat water bath. The specimen coins were submersion in the investigate solution without and with different doses of the tested compound.
All test solutions are opened to air. Through 180 minutes, the specimens were taken out, washed, dried, and weighed accurately per half an hour. The mean (WL) for seven square CS coin samples will be obtained.
The degree of coverage (θ) and the (% IE) of Gliclazide inhibitor for the oxidation of CS were determinate as follows [32]:
Where, Wº and W are (WL), in nonexistence and existence the different concentration of the investigate drug compound respectively.
2.3.2. Gasometric measurements
Measurements of hydrogen evolutions were estimation at 25oC, and the hydrogen volume developed every 15 minutes, (Ɵ) and the (% IE) were determinate by (3) and (4).
Where, V is the volume of hydrogen in cm3, K is rate constant and t is time in minute.
Where, Ko and K are the rate constant of corrosion in nonexistence and existence drug, which determinate by plotting V vs. t and K value is the slope.
2.3.3. Potentiodynamic polarization technique
The polarization cell comprise of three poles are (SCE) terminal that coupled to the fine Luggin hairlike as the reference pole, platinum counter pole and working anode pole. The working terminal is a square cut from CS sheet settled by epoxy pitch so that the level surface zone was 1.0 cm2. The working electrode was polisher with SiC papers grit 1200 in size. The measurements were taken after the electrodes submersion in corrosive medium at natural potential for 10 minutes until reach the steady state. The (Eocp) technique was started from – 533 to – 475.5 mV. All coin samples were achieves in new prepared solutions at 25oC and data were always worked again to check the validity results. The (θ) and the (% IE) were determinate by the relation (5) [33]:
Where, icorr(free) and icorr(inh) are the current densities of corrosion in the nonexistence and existence of drug, respectively.
2.3.4. Electrochemical Impedance
Spectroscopy (EIS) technique They got distances or diameters across of the capacitive circles increment in nearness of medication, and are demonstrative of the capacitive of the degree of inhibits of erosion process, in spite of the decline or reduced of the limit of twofold layer (Cdl) which is characterized as:
Where, fmax is the maximum frequency.
The (%IE) and the (θ) given from the (EIS) data were determinate by the relation:
Where, Rop and Rp are the resistance of charge transfer in the nonexistence and existence of drug, sequence.
2.3.5. Electrochemical Frequency Modulation (EFM) technique
The measurements of (EFM) were achieved by implementation potential concerned signal with abundance 10 mV with two sine waves of 2 and 5 Hz. The frequencies that choice are 2 and 5Hz depend on three arguments [34]. The corrosion current density (icorr) was determinate from the two larger peaks and the Tafel slopes (βc and βa) and the causality factors CF2 and CF3 [35]. The (%IEEFM) was determinate by applied the follows equation:
Where, iocorr and icorr are corrosion current densities in the nonexistence and existence of drug, respectively.
2.3.6. Surface Examinations
The CS coins used for analysis of morphology surface were prepared in 1M HCl acid (blank) and with 300 ppm of Gliclazide at 25oC for 24 hours after polisher mechanically by utilizing various emery papers up to 1000 and 1500 grit size. Then, after this submersion time, the coin samples were clean carefully by bi-distilled water, gently dried and achieves the coin samples examined by using scanning electron microscope (SEM), (EDX) and (AFM).
3. Results and discussion
3.1. (WL) measurement
The (WL) of CS in mg cm-2 relative to the surface area at different time periods in the nonexistence and existence of various doses (50 ppm -300 ppm) of the Gliclazide were determination. The obtained curve in the presence of various doses of drugs drown below that of free acid as seen in Figure 1.

Fig. 1: The bends lines or curves of (WL)- time for the oxidation of CS in the nonexistence and existence of various dosages of gliclazide at 25oC
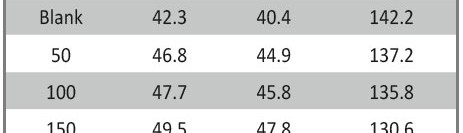
The (% IE) are recorded in (Tab. 3). In all state, the (% IE) of the drug increases with increasing doses of drug but the rate of corrosion were decreasing. These data indicated that, the Gliclazide as drugs under testing are good efficiency for CS oxidation in HCl medium.

Tab. 3: The (% IE) variation of Gliclazide with various doses at 25oC from (WL) data at 120 minutes submersion in 1 M HCl medium
3.1.1. Effect of temperature
Using Arrhenius equation to study the influence of temperature and rate constant (kcorr) to determination of activation energy:
Where, R is the general gas constant, Ea is the activation energy, T is the (Ko) and A is a Arrhenius pre-exponential constant based on electrolyte and the nature of metal. When plotting the log kcorr. against (1/T) for CS in 1 M HCl in the nonexistence and existence of various doses of Gliclazide are seen in diagram in Figure 2. Gives straight lines that have slope (-Ea/2.303R) and the values of Ea were determinate and recorded in (Tab. 4). It is obvious that the drug behavior has the same of action mechanism. The activation energy (Ea) increases with the adding of various doses of drug, lead to, the increased of the energy barrier of the oxidation reaction and controlled the whole process by surface reaction, since the activation energy over 20 kJ mol-1 [36].

Fig. 2: Arrhenius draw (log k against 1/T) for corrosion of CS in 1M HCl in the nonexistence and existence of various doses measurements of Gliclazide
The entropy and enthalpy of activation (βS*, βH*) are determinate from the theory of transition theory by applied the follows relation [37].
Where, N is Avogadro’s number h is Plank’s constant. Draw log (kcorr./T) vs. (1/T) also gave straight lines as seen in Figure 3, for CS that oxidation in 1M HCl in the nonexistence and existence of various doses of Gliclazide. The (-βH*/2.303R) are the slopes of these lines and the [log [RT/Nh] + (βS*/2.303R)] is intercept, that the values of (βH*) and (βS*) were determinate and recorded in (Tab. 4). From these data, it is obvious that the available of the test compound rising the values of (Ea) and follow reducing the rate of corrosion of the CS. From these data lead to the lest compound behavior as an inhibitor as a result the increasing Ea of CS oxidation by transfer of charge for their adsorption on the CS surface and making thin film barrier. The values of βH* reflects the strong adsorption of Gliclazide compound on CS surface. The estimations of βS* in nonexistence and existence of the investigation compound is negative and substantial esteem values, this prompt the rate-deciding of initiated complex represents to an collection step, instead of separation step, implying that a reductions in irregular happens and foreword from the enacted or activated complex of the reactants and put the actuated particles in more request state than that at the underlying or initial state [38].

Fig. 3: Draw of (logkcorr. / T) vs. (1/ T) for corrosion of CS in 1 M HCl in the nonexistence and existence of various doses measurements of Gliclazide at 25oC

Tab. 4: Thermodynamic variables for the dissolution of CS in 1 M HCl in the nonexistence and existence of varied doses measurements of investigated drug
3.1.2. Adsorption isotherm
Assuming the inhibition of corrosion due to the adsorption of Gliclazide, and the values of (θ) for various doses of drug in 1 M HCl was determinate from (WL) data utilizing the follows relation:
The surface coverage (θ) increases with increasing the doses of the tested (Gliclazide) drug inhibitor. It’s utilized in Langmuir adsorption isotherm that obeyed with experimental data that found fit on it. The mathematical expression of Langmuir is given as following [39].
Where, kads. is the equilibrium constant of adsorption. Plotting (C/Ө) vs. (C) of Gliclazide at various temperatures is introduced in Figure 4 recommends that no forces repulsion or attraction between the atoms adsorbed, ever after relationship a linear is given with intercept equal to (1/kasd.) and slope similar the unity, the adsorption constant being result to the standard free energy of ΔGoads adsorption by equation:
Where, R is the general gas constant, T is (Ko) and 55.5 is the doses of water in M/L. The values of ΔGoads at all studied temperatures which determinate by above equation (13) and recorded in (Tab. 5). The heat of adsorption (ΔHoads.) was determinate according to the Van’t Hoff relation [40].
Plotting (logkads) versus (1/T) give straight line that seen in Figure 5, the straight line gives slope equal (ΔHoads./2.303R), from this slope, the ΔHoads were determinate and listing in (Tab. 5). Then in accordance with the basic equation (10):
From introducing the values of ΔGoads and ΔHoads., the ΔSoads was determinate at all studied temperatures by the above equation (15). All thermodynamic adsorption parameters for Gliclazide drug on CS from 1M HCl solution can be concluded that:
- The correlation coefficients between (0.99 – 0.98) reflected the experimental data which gives a good curves that exactly for the implementation adsorption isotherm.
- Kads. values increases with increasing temperatures from 30 to 45oC except at 25oC.
- The negative values of ΔGoads reflected that the adsorption of gliclazide on CS surface in 1 M HCl medium is spontaneous process.
- ΔGoads slightly increases (becomes less negative) with increasing temperatures which indicated that the occurrence of endothermic process and the adsorption was unfavorable with increasing temperature of reaction due to decreasing the electrostatic attraction of the drug desorption with the surface of CS [41].
- The ΔGoads values are around -20 kJ mol-1 or less lead to the electrostatic attraction between positive charged of metal surface and the negative charge of organic molecules in the bulk of the medium i.e. physical adsorption.
- The positive sign of ΔHo ads refer to the adsorption of drug compound is an endothermic process, lead to the physical adsorption. The unshared electron pairs in investigate molecule may attractive with positive center on the surface of CS by electrostatic attraction to provide a protective film prevent corrosion process [42].
- The ΔSo ads values, in the existence of the investigate drug are positive and large that is accompanied with endothermic adsorption process [43].
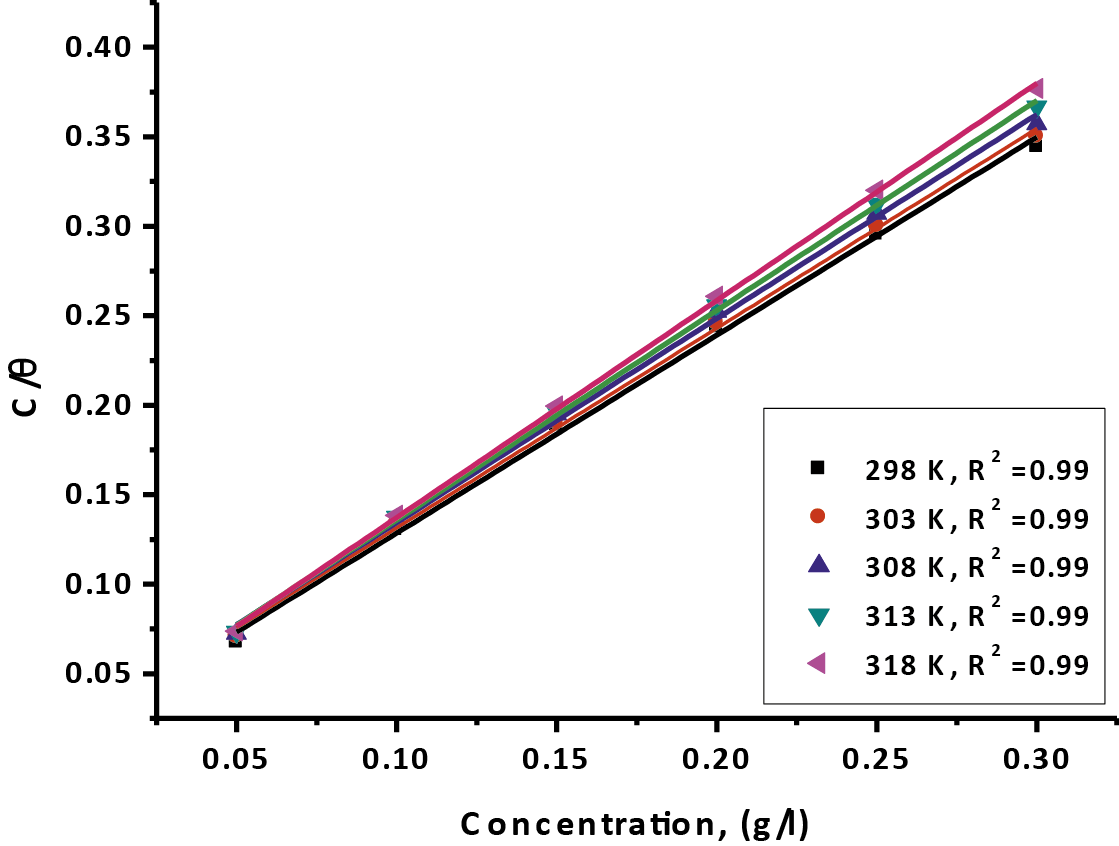
Fig. 4: Plotted the Langmuir adsorption isotherm as (log C) against (C/θ) of the tested drug for oxidation of CS in 1 M HCl solution from (WL) technique at various temperatures.

Tab. 5: The (Kads) and free energy (ΔG˚ads) for the adsorption of Gliclazide on CS in 1 M HCl from (WL) technique at various temperatures
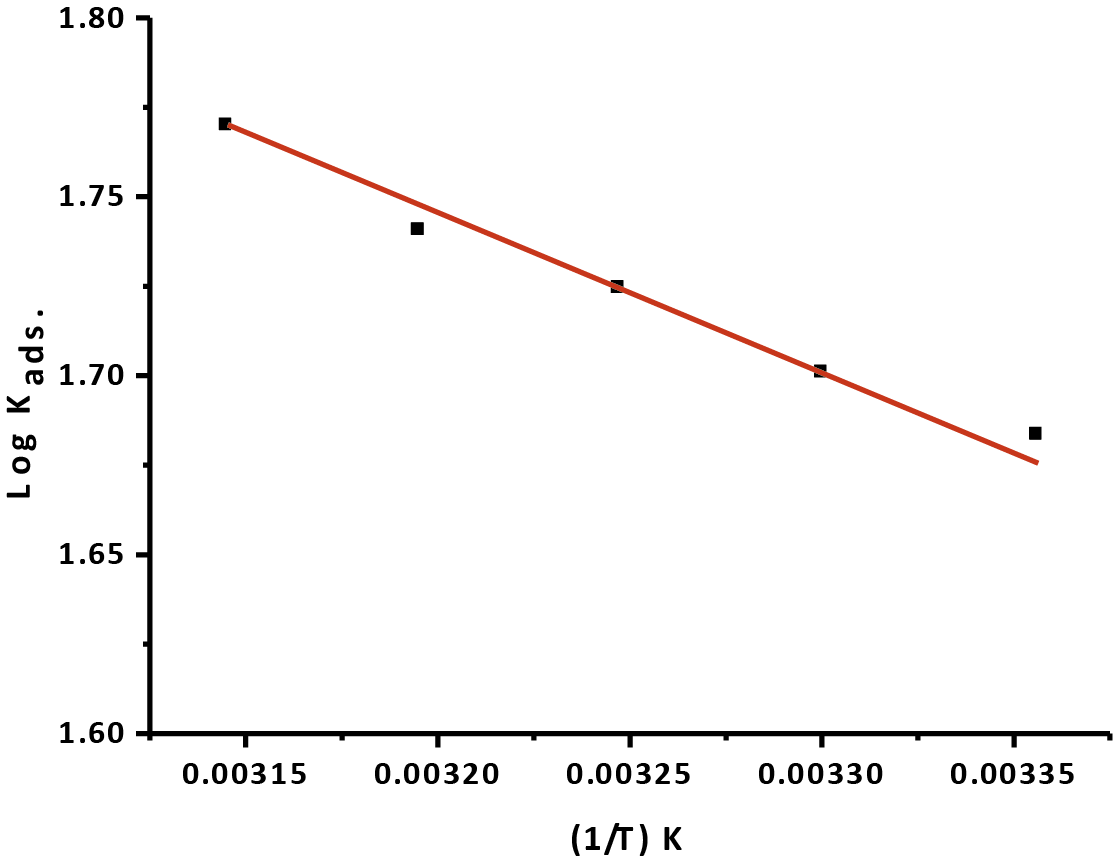
Fig. 5: (log kads.) vs. (1/T) for the oxidation of CS in 1M HCl in the existence of Gliclazide at various absolute temperatures
3.2. Hydrogen Evaluation (HE)
All information drowns from the volume of hydrogen which produces at versus time, for 50 – 300 ppm of Gliclazide focuses and exhibited in Figure 6. The slope of line evaluated the rate of corrosion. The great straight lines show the insoluble film on the metal surface. The certain of the rate of corrosion acquired from hydrogen evaluation individually at versus concentration are recorded in (Tab. 6). As shown, the rate of corrosion reduced with increasing of Gliclazide concentration, appearing diminishes conduct for the metal disintegration. This result is normal on the grounds that with increasing drug, both acidity and Cl- ion focus are lessening. As indicated by chemical equation (11) pointed out that Fe dissociation in acid arrangements relies on hydrogen ion more than the chloride ion [44]. H+ advancement and mass misfortune is delivered by the same response:
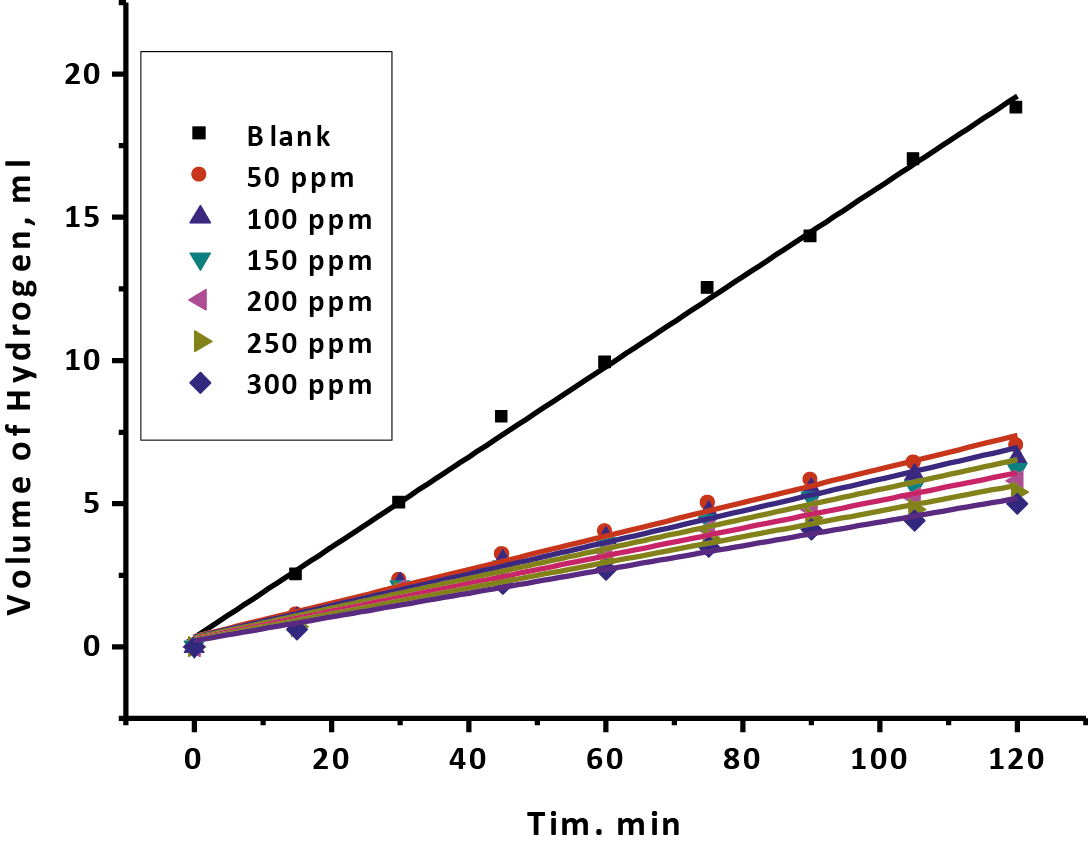
Fig. 6: Hydrogen volume produced versus time arrangements with distinctive centralization of drug at 25oC
3.3. Open circuit potential (EOCP)
From the Figure 7 and (Tab. 7) is shown several interesting points:
- The EOPC in the blank solution is beginning from -533 mV then shifted anodically and reached the steady state after 300 S indicating that the initial dissolution process and formation oxide film on the surface of the metal.
- The EOCP is started in the existence of Gliclazide, at less negatively potential compared with that in the nonexistence of the drug and then shifted anodically that starting from 500.8, 495.3, 487.2, 479.4, 476.2 and 475.5 according to the increasing the concentration 50, 100, 150, 200, 250 and 300 respectively. The steady state is attained rapidly, with increasing the doses of the drug comparing with the blank, then the shift in the potential of EOCP increasing in the active direction pointing and the drug might certain act mainly as an anodic inhibitor [45]. However, from Figure 7, the shifts in EOCP on add Gliclazide inhibitor is 57.5 mV revealing that the existence drug acts as anodically drug.

Tab. 7: Open circuit potential of the CS in nonexistence and in existence of Gliclazide drug at 25oC.

Fig. 7: The curves illustrated the of open circuit potential (EOCP) for CS that submersion in 1M HCl in the nonexistence and existence of Gliclazide drug at 25oC
3.4. Potentiodynamic polarization (PP)
Anodic and cathodic polarizations were carried out potentiodynamic in 1 M HCl medium in the nonexistence and existence of different doses of Gliclazide at 25oC. The results are drowning in Figure 8. The obtained values of potentiodynamic polarization parameters are recorded in (Tab. 8). The cathodic and anodic bend lines or curves are acquired by Tafel-sort conduct. The type of the bend lines or curves are fundamentally the same and closed to gather, which lead to the mechanisms of CS oxidation and hydrogen reduction apparently remain in the existence Gliclazide drug. Both the cathodic and anodic current den-sities reduced when addition different concentration of Gliclazide and made chiefly parallel uprooting the more positive and negative values individually. This mean the existence of Gliclazide in medium inhibit both the anodic dissolution processes and the hydrogen evolution with overall shift of Ecorr to more negative values.
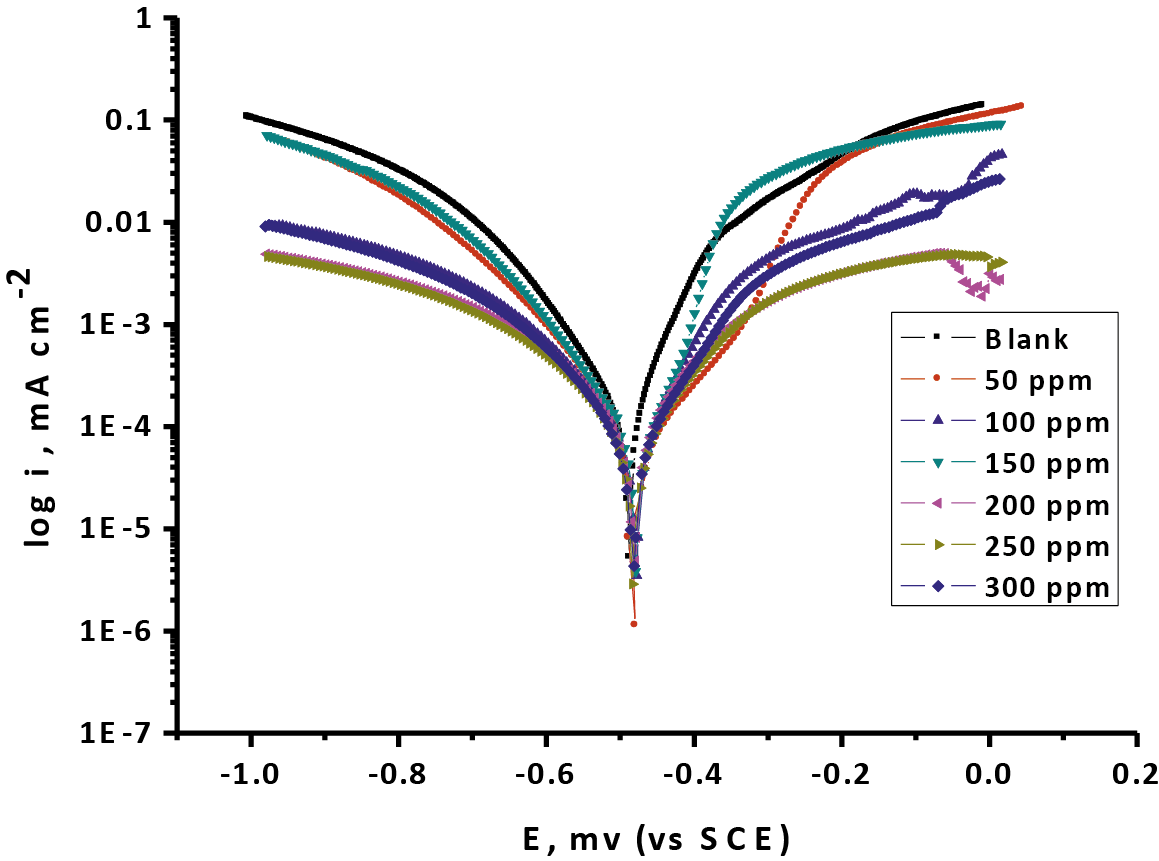
Fig. 8: The (PP) bend lines or curves for the oxidation of CS in 1 M HCl in the nonexistence and existence of varied doses of Gliclazide at 25°C
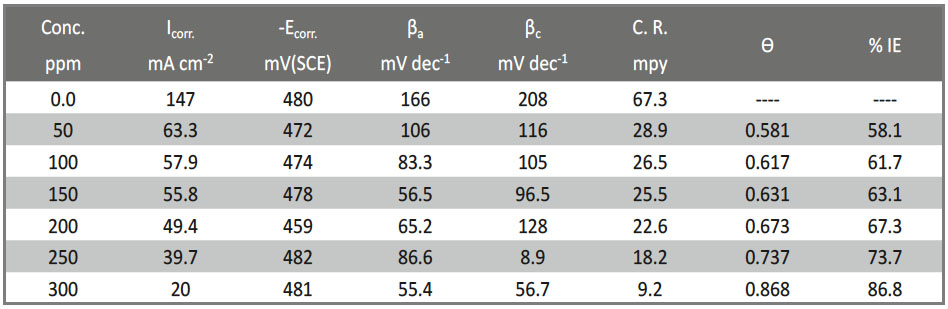
Tab. 8: The influence of concentration of Gliclazide on the (Ecorr), (icorr), Tafel inclines (βa& βc), (% IE) and (θ) of CS in 1 M HCl at 25oC
The outcomes likewise are demonstrated that the anodic and the cathodic (βa and βc) marginally or slightly changed with expanding the dosages of the tested Gliclazide compound. This lead to there is no change of the mechanism of restraint in nearness and nonattendance of medications. In the fact the values of (βc) are slightly more than the values of (βa) refer to the cathodic action of the drug. It is obvious that an action of mixed drug control over the electrochemical semi-reactions. Clearly an activity of blended medication control over the electrochemical semi-responses. This implies the Gliclazide is blended sort medicate, yet the cathode is more specially spellbound than the anode. The higher estimations of Tafel incline line or slope can be identified with the surface motor or kinetic of surface process instead of the dispersion controlled process [46]. The cathodic inclines or slopes are gotten from the (PP) estimations demonstrate that the hydrogen advancement response was actuation controlled [47] and the expansion of the Gliclazide medicate did not alter the system of this procedure or mechanism. This outcome creates the impression that the inhibit method of the Gliclazide was utilized by straightforward follows of the surface by adsorption prepare.
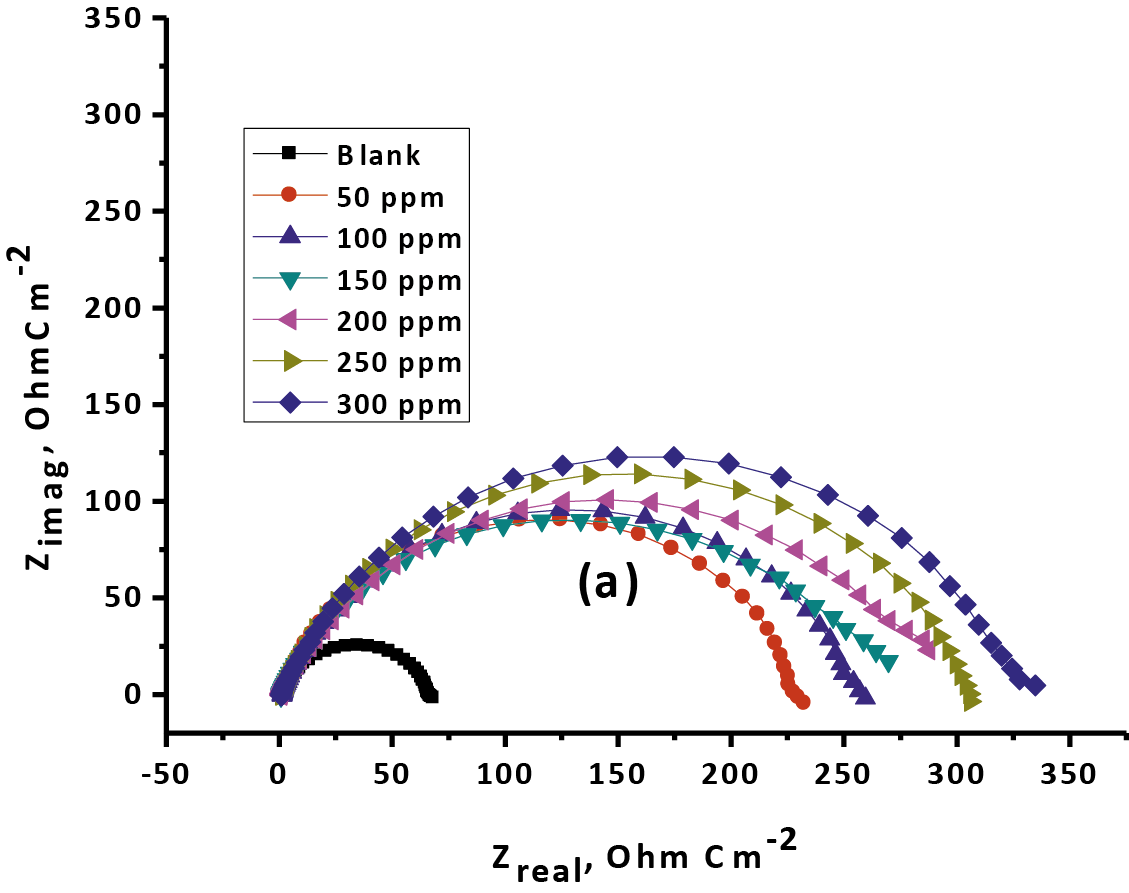
3.5. Electrochemical Impedance Spectroscopy (EIS)
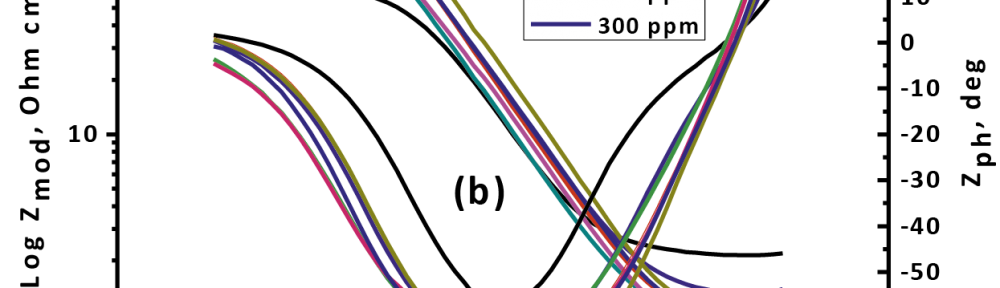
The (EIS) charts (Nyquist and bode) at frequencies extending from 0.1 Hz to 105 Hz with 10 mV plenitude motion at OCP for CS in 1 M HCl in the nonexistence and existence of varied measurements of Gliclazide doses are acquired. The identical circuit that depict for (CS) and electrolyte are found in Figure 9, where Rs, and Rp allude to arrangement resistance and charge exchange resistance, separately. EIS parameters and (% IE) were determinate and recorded in (Tab. 9).

Tab. 9: The variables that obtained by EIS method for CS in 1M HCl without and with varied doses of Gliclazide at 25 oC
The obtained Nyquist and bode plotting for Gliclazide is seen in Figure 10. Nyquist spectrum is characterized by a single full half-circle. These seen that the corrosion of CS is controlled by a charge exchange prepare [48]. The widths or diameter of the capacitance circle got that increments within the sight of Gliclazide were demonstrated that the increases or ascents the (% IE) of the consumption procedure [49].

Fig. 10: The Nyquist (a) and Bode (b) curves for oxidation of CS in 1 M HCl in the nonexistence and existence of various doses of Gliclazide at 25 °C
From the results of (EIS) that obtained Rp rises and Cdl reducing with increasing of Gliclazide drug doses. The ascent or rise in Rp values improve the increases or ascents of the %IE because of the progressive substituent of water particles by the adsorption of the medication particles on the metal surface by an adherence film form on the metal surface. The formation film on the metal surface reduced the double layer thickness. Also, the decreasing of Cdl with rises the drug doses as result from reduce in local dielectric constant which indicating that, the drug was adsorbed on anodic sites and covered the cathodic sites on the surface of the metal [50].
3.6. Electrochemical Frequency Modulation technique (EFM)
The (EFM) technique is defined as a nondestructive corrosion measurement and a very good technique for determination corrosion information’s [51].
The CF-2 and CF-3 are determinate or estimate from the range of the present spectrum of the reactions and the quality of the (EFM) is the causality components which fill in as an interior keep an eye on the good of EFM estimation. The EFM Inter-modulation spectrums of CS in 1 M HCl acid medium and in 1M HCl with containing (50 ppm – 300 ppm) of the Gliclazide are seen in Fig. 11. The intermodulation peaks and harmonic are obviously visible and are much larger than the background noise. The two high crests, with plenitude of about (<100 μA), are the reaction to the 100 mHz (2 and 5 Hz) excitation frequencies. It is essential to note that between the tops of crests there is almost no present reaction or current response (<100 μA). The information of (EFM) was dealt with by using two different models: the “activation” model demonstrates and finishes or completes dissemination control of the cathodic response. For the latter, a set of the solution of three nonlinear equations, suppose that the corrosion potential does not change as a result of the polarization of the working electrode [52]. The highest crests or pinnacles were usage to estimate (jcorr), the causality variables (CF-2 and CF-3) and Tafel inclines or slopes (βa and βc). At the same time, the electrochemical variables were assurance by Gamry EFM140 programming, and recorded in (Tab. 10). The data, obviously show that, add of investigated drug compound at a given doses to the acidic medium reducing the corrosion current density, lead to the Gliclazide frustrate or inhibit the corrosion of CS in 1 M HCl through adsorption. The causality components got under various trial conditions are approach equivalent to the hypothetical qualities (2 and 3) demonstrating that the estimation information are genuine and of good quality [53]. The (% IE) EFM are increased by rises the doses of Gliclazide which determinate and recorded in (Tab. 10).
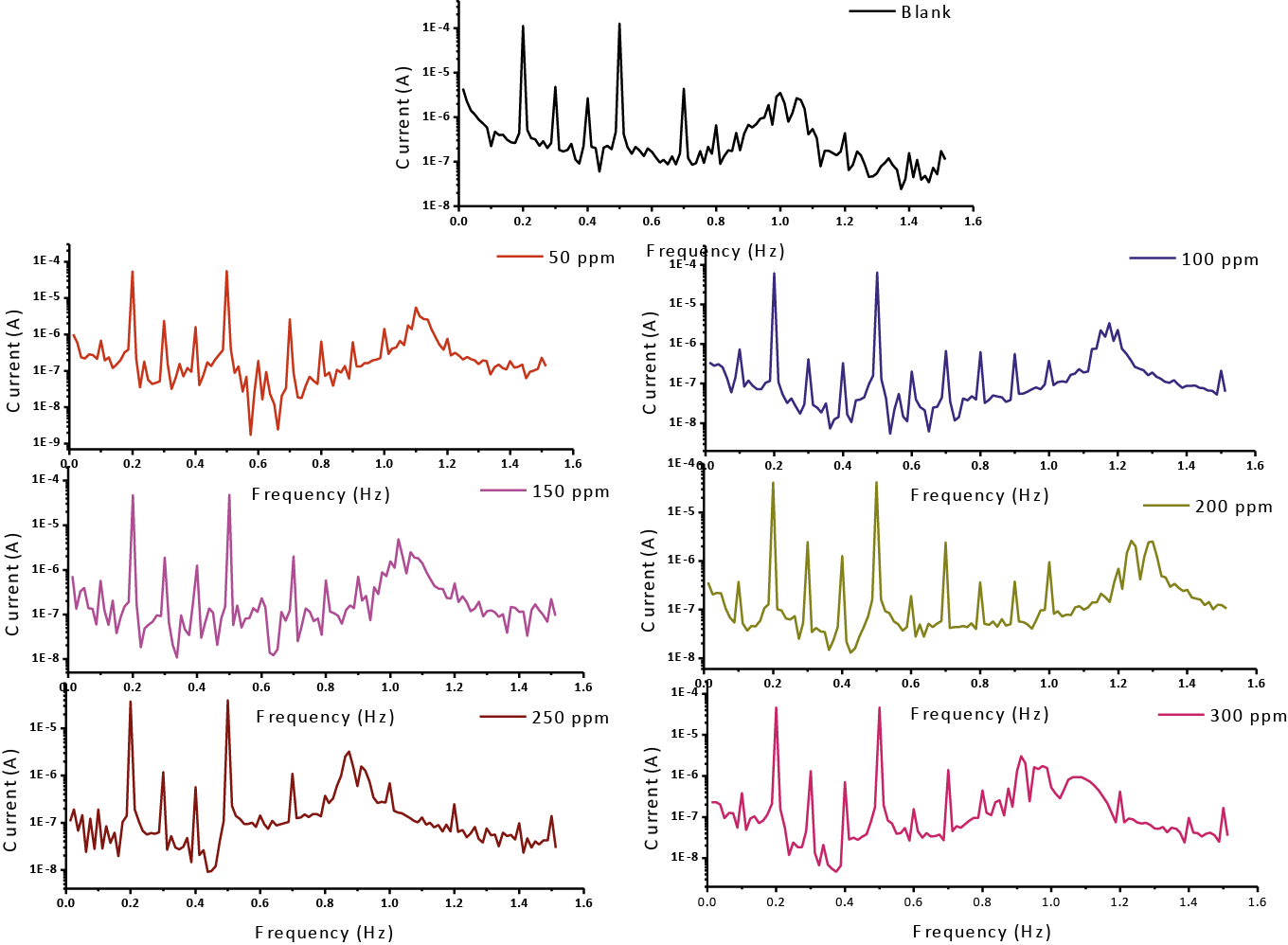
Fig. 11: EFM for meal in 1M HCl unlucky deficiency and vicinity of distinctive convergences of Gliclazide

Tab. 10: EFM kinetic variables calculation from CS that submersion in 1 M HCl without and with various doses of Gliclazide at 25 oC
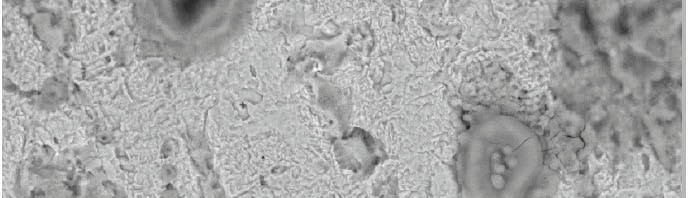
3.7. Scanning Electron Microscopy (SEM)
Figure 12, represents the micrograph obtained for CS specimens in nonexistence and existence of 300 ppm of Gliclazide after exposure for 1 day submersion. It is obvious that CS surfaces help and facilitate corrosion attack in the blank sample.

Fig. 12: SEM microstructures for CS in the nonexistence and
existence of 300 ppm of Gliclazide after submersion for 1 day
It is significant to worry that when the compound is available in the solution, the morphology of CS surfaces is very unique in relation to the past one and the specimen coin surface was smoother. We noticed the make thin film which is circulated arbitrarily in general surface of the CS This might be thus the adsorption of the Gliclazide on the CS surface and make the inactive film keeping in mind the end goal to hinder the dynamic site show on the CS surface. The drug molecule interaction with active sites of CS surface, that resulting the reducing in the contact between CS and the corrosive solution and sequentially exhibited excellent inhibition effect [54-55].
3.8. Energy Dispersion Spectroscopy (EDX)
The EDX spectra were utilized to determine the elements existence on the surface of CS and after 1 day of immersion in acid with optimum concentration of drug. Figure 13, award to the EDX examination of CS in 1 M HCl with within the sight of 300 ppm of Gliclazide. The spectrum demonstrates extra lines, showing the existence of C (attributable to the carbon molecules of some Gliclazide). These data shows that the carbon, nitrogen, oxygen and sulfur atoms covered the specimen surface. The EDX analysis indicates that only nitrogen, carbon, oxygen and sulfur were detected, and show that the passivation film contained the chemical formula of Gliclazide drag adsorbed on the surface of CS. It is seen that, the percent weight of adsorb elements N, C, O and S were present in the spectra and recorded in (Tab. 11).

Tab. 11: Surface composition (% weight) of CS after one day of submersion in 1M HCl nonexistence and existence the 300 ppm of Gliclazide
3.9. Atomic Force Microscopy (AFM)
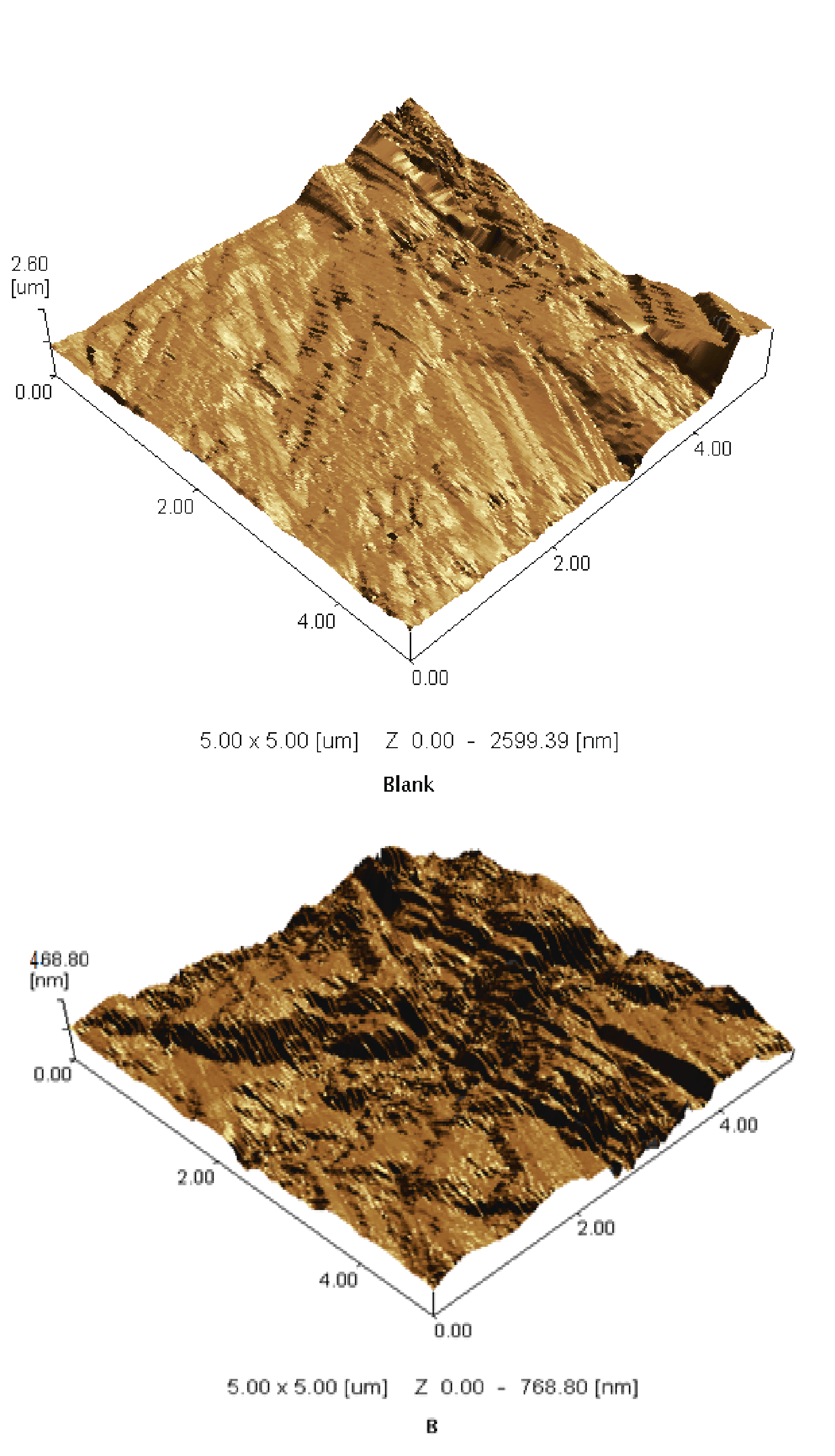
AFM is a powerful tool to investigate the surface morphology of various samples at nano- micro scale that is currently used to study the influence of corrosion drugs on metal solution interface. From the analysis, it can be gained regarding the roughness on the surface. The roughness profile values play an important role in identifying and report the efficiency of the drug under study. Among the roughness take a role in explanation about the nature of the adsorbed film on the surface [45-46]. Figure 14, shows the 3D images as well as elevation profiles of polished of CS in absence and present Gliclazide as drug. It observed in Figure 14, the surface of CS specimen (a) exposed to corroded solution affected vales structure with large and deep crack but the surface (b) reveal that is covering film adsorbed on the metal surface. The conclusion, that the adsorption film can protect the surface of the metal from corrosion process. Analysis of the values indicated higher the values of roughness parameter reached. The mean roughness is found to be (2.60 μm) for the blank in acid solution which placed in 1M HCl one dye and analyzed. The observation of the metal surface which immersed in 1M HCl in existence of 300 ppm of Gliclazide drug possess roughness (468.80 nm) compared to the blank solution. It can be noted that the value is lower than that of the blank value. The decrease in the roughness value reflected to the adsorption of drug molecule on metal surface thereby reducing the rate of corrosion.
4. References
- Trabanelli G., drugs – an old remedy for a new challenge Corrosion, 1991, 47, 410-419.
- Singh D.N. and Dey A.K., Synergistic Effects of Inorganic and Organic Cations on Inhibitive Performance of Propargyl Alcohol on Steel Dissolution in Boiling Hydrochloric Acid Solution Corrosion, 1993, 49, 594 – 600.
- Banerjee G. and Malhotra S.N., Contribution to the adsorption of aromatic amines on mild steel surfaces from HCl solutions by impedance, UV and Raman spectroscopy Corrosion-NACE, 1992, 48, 10 – 15.
- Arab S.T. and Noor E.A., Inhibition of Acid Corrosion of Steel by Some S-Alkylisothiouronium Iodides, Corrosion, 1993, 49,122 – 129.
- Raspini I. A., Influence of Sodium Salts of Organic Acids as Additives on Localized Corrosion of Aluminum and Its Alloys, Corrosion, 1993, 49, 821-828.
- Hajjaji N., Ricco I., Srhiri A., Lattes A., Soufiaoui M., Benbachir A., Effect of N-Alkylbetaines on the Corrosion of Iron in 1 M HCl Solution, Corrosion, 1993,49, 326-334.
- Elachouri M., Hajji M.S., Salem M., Kertit S., Coudert R., Essassi E.M., Some surfactants in the series of 2-(alkyldimethylammonio) alkanol bromides as drugs of the corrosion of iron in acid chloride solution Corros.Sci., 1995, 37, 381-389.
- Luo H., Guan Y.C., Han K.N., Inhibition of mild steel corrosion by sodium dodecyl benzene sulfonate … and Sodium Oleate in Acidic Solutions, Corrosion, 1998, 54, 619-627.
- Migahed M.A., Azzam E,M,S., Al-Sabagh A.M., Corrosion inhibition of mild steel in 1 M sulfuric acid solution using anionic surfactant Mater.Chem.Phys., 2004, 85, 273-279.
- Osman M.M., Omar A.M., Al-Sabagh A.M., Corrosion inhibition of benzyl triethanol ammonium chloride and its ethoxylate on steel in sulphuric acid solution Mater.Chem. Phys., 1997, 50, 271- 274.
- Zucchi F., Trabanelli G., Brunoro G., The influence of the chromium content on the inhibitive efficiency of some organic compounds Corros.Sci., 1992, 33, 1135-1139.
- Villamil R.F.V., Corio P., Rubim J.C., Siliva Agostinho M.L., Effect of sodium dodecylsulfate on copper corrosion in sulfuric acid media in the nonexistence and existence of benzotriazole, J.Electroanal.Chem., 1999, 472 ,112-119.
- Zhao T.P., Mu G.N., The adsorption and corrosion inhibition of anion surfactants on aluminium surface in hydrochloric acid Corros.Sci., 1999, 41, 1937-1944.
- Abd El Rehim S.S., Hassan H., Amin M,A., Corrosion inhibition of aluminum by 1,1(lauryl amido)propyl ammonium chloride in HCl solution Mater.Chem.Phys., 2001, 70, 64 – 72.
- Abd El Rehim S.S., Hassan H., Amin M,A., The corrosion inhibition study of sodium dodecyl benzene sulphonate to aluminium and its alloys in 1.0 M HCl solution Mater.Chem. Phys., 2003, 78, 337-348.
- Guo R., Liu T., Wei X., Effects of SDS and some alcohols on the inhibition efficiency of corrosion for nickel, Colloids Surf., A, 2002, 209, 37-45.
- Branzoi V., Golgovici F., Branzoi F., Aluminium corrosion in hydrochloric acid solutions and the effect of some organic drugs, Mater.Chem.Phys., 2002, 78, 122-131.
- Oukhrib R., El Ibrahimi B., Bourzi H., El Mouaden K., Jmiai A., El Issami S., Bammou L., Bazzi L.; Quantum chemical calculations and corrosion inhibition efficiency of biopolymer “chitosan” on copper surface in 3%NaCl, JMES, 2017, 8 (1): 195-208.
- Al-Azzawi A. M. and Hammud K. K., Newly antibacterial / anti-rusting oxadiazoleporomellitic di-imids of carbon steel / hydrochloric acid interface: Temkin isother model, IJRPC, 2016, 6(3): 391-402.
- El Ouasif L., Merimi I., Zarrok H., El ghoul M., Achour R., Guenbour M., Oudda H., El-Hajjaji F. and Hammouti B.; Synthesis and inhibition study of carbon steel corrosion in hydrochloric acid of a new surfactant derived from 2-mercaptobenzimidazole , J. Mater. Environ. Sci., 2016,7 (8): 2718-2730.
- Sani U. M. and Usman U.; Electrochemical Corrosion Inhibition of Mild Steel in Hydrochloric Acid Medium Using the Antidiabetic Drug Janumet as Drug , International Journal of Novel Research in Physics Chemistry & Mathematics, 2016, 3(3): 30-37.
- Kolo A.M., Sani U.M., Kutama U. and Usman U.; The Pharmaceutical and Chemical Journal, 2016, 3(1):109-119.
- Ameh P. O. and Sani U. M.; Cefuroxime Axetil: A Commercially Available Pro-Drug as Corrosion Drug for Aluminum in Hydrochloric Acid Solution ,Journal of Heterocyclics, 2015, 1(1): 2 – 6.
- Al-Shafey H. I., Abdel Hameed R. S., Ali F. A., Aboul-Magd A. S., Salah M.; Effect of Expired Drugs as Corrosion Drugs for Carbon Steel in 1M HCL Solution, Int. J. Pharm. Sci. Rev. Res., 2014, 27(1):146-152.
- Kushwah R. and Pathak R. K.; Inhibition of Mild Steel Corrosion in 0.5 M Sulphuric Acid Solution by Aspirin Drug, International Journal of Emerging Technology and Advanced Engineering, 2014, 4(7): 880-884.
- Fouda A. S., EL-Haddad M.N. and Abdallah Y.M.; Septazole: Antibacterial Drug as a Green Corrosion Drug for Copper in Hydrochloric Acid Solutions,IJIRSET, 2013, 2 (12): 7073-7085.
- Ofoegbu S. U. and Ofoegbu P. U.; Corrosion inhibition of mild steel in 0.1 M hydrochloric acid media by chloroquine diphosphate , ARPN Journal of Engineering and Applied Sciences, 2012, 7 (3): 272-276.
- Bentiss Traisnel F., Lagrenee M., The substituted 1,3,4-oxadiazoles: a new class of corrosion drugs of mild steel in acidic media, Corros.Sci., 2000, 42, 127-146.
- Christopher M.A.B., Isabel Jenny A.R.G., The electrochemical behaviour and corrosion of aluminium in chloride media. The effect of drug anions, Corros.Sci., 1994, 36, 915-923.
- Elachouri M., Hajji M.S., Salem M., Kertit S., Aride J., Coudert R., Essassi E., Some Nonionic Surfactants as Drugs of the Corrosion of Iron in Acid Chloride Solutions, Corrosion, 1996, 52,103-108.
- Algaber A.S., El-Nemma E.M., Saleh M.M., Effect of octylphenol polyethylene oxide on the corrosion inhibition of steel in 0.5 M H2SO4, Mater.Chem.Phys., 2004, 86, 26-32.
- Mu G.N., Zhao T.P., Liu M., Gu T., Effect of Metallic Cations on Corrosion Inhibition of an Anionic Surfactant for Mild Steel , Corrosion Sci., 1996, 52, 853-856.
- Lipkowski J., Ross (Eds.)P. N., Adsorption of Molecules at Metal Electrodes, VCH, New York, 1992.
- Da Costa S. L. F. A. and Agostinho S. M. L., Electrochemical studies of cu-Al Alloys in Sulphate – SciELO, Corrosion, 1989, 45, 472 – 477.
- Aljourani J., Raeissi K., Golozar M.A., Benzimidazole and its derivatives as corrosion drugs for mild steel in 1M HCl solution, Corros. Sci., 2009, 51 ,1836-1843.
- Amar H., Tounsi A., Makayssi A., Derja A., Benzakour J., Outzourhit A., Corrosion inhibition of Armco iron by 2-mercaptobenzimidazole in sodium chloride 3% media Corros. Sci., 2007, 49, 2936-2945.
- Migahed M.A., Azzam E.M.S., Morsy S.M.I., Electrochemical behaviour of carbon steel in acid chloride solution in the presence of dodecyl cysteine hydrochloride self-assembled on gold nanoparticles Corros.Sci., 2009, 51, 1636-1644.
- Bllglc S., Caliskan N., An investigation of some Schiff bases as corrosion drugs for austenitic chromium-nikel steel in H2SO4, Applied Electro-chemistry, 2001, 31, 79-83.
- Ashassi-Sorkhabi H., , Ghalebsaz-Jeddi N., Inhibition effect of polyethylene glycol on the corrosion of carbon steel in sulphuric acid , Mater.Chem.Phys., 2005, 92, 480-486
- El Maghraby A., Soror T. Y., Efficient surfactant as corrosion drug for carbon steel in hydrochloric acid solutions, Adv. App. Scie., 2010, 1, 156 – 168.
- Khalifa O. R., Abdallah S. M., Corrosion inhibition of some organic compounds on low carbon steel in Hydrochloric acid solution, Portugaliae Electrochemica Acta, 2011, 29, 47-56.
- Al-shafey I., Abass M. A., Hassan A. A., Sadeek S. A., Corrosion inhibition of carbon steel in 1M HCl Solution by Schiff base compound obtained from 1,3-Diaminopropane, IJABC, 2014, 3, 1004-1015.
- Fouda A. S., Gouda M. M., and Abd El-Rahman S. I., Benzaldehyde, 2-Hydroxybenzoyl Hydrazone Derivatives as Drugs of the corrosion of Aluminium in hydrochloric Acid, Chem. Pharm. Bull., 2000, 48 (5), 636- 640.
- Fouda A, S., El-Wakeel A. M., Shalabi K., El-Hossiany A., Corrosion inhibition for carbon Steel by Levofloxacin Drug in Acidic Medium, Elixir Corrosion &Day, 2015, 83, 33086-33094.
- Hazazi O. A., Fawzy A., Awad M.; Synergistic Effect of Halides on the Corrosion Inhibition of Mild Steel in H2SO4 by a Triazole Derivative: Kinetics and Thermodynamic Studies, Int. J. Electrochem. Sci., 2014, 9, 4086 – 4103.
- Fouda A. S., El- Defrawy A. M. and El-Sherbeni M. W., Pharmaceutical compounds as save corrosion drugs for carbon steel in 1M H2SO4 solution, Reprint form the Mansoura, J, Chemistry, 2012, 39(2) 1-27.
- Fouda A.S., , Al-Sarawy A.A., El-Katori E.E., Pyrazolone derivatives as corrosion drugs for Mild steel HCl solution, Desalination, 2006, 201 ,1-13.
- Farag A. A., Ibrahim I. M., Influence of Nonionic Surfactant on the Carbon Steel Corrosion in Hydrochloric Acid Solution, IJSR, 2014, 3, 1087-1091.
- Benalli O., Larabi L., Traisnel M., Gengembra L., Harek Y., Electrochemical, theoretical and XPS studies of 2-mercapto-1-methylimidazole adsorption on mild steelin 1 M HClO4, Appl. Surf. Sci., 2007, 253, 6130-6139.
- Kshama Shetty S., Nityananda A., Shetty, Ionic Liquid as an Effective Corrosion drug on 6061Al-15 Vol.PCT.SIC(p) Composite in 0.1M H2SO4 Medium, An Ecofriendly Approach, 2015, 3, 41-64.
- Kus E., Mansfeld F.,an Evalation of the Electrochemical Frequency Modulation (EFM) Technique, Corros. Sci. 2006, 48, 965-979.
- Caigman G. A., Metcalf S. K., Holt E. M., Thiophene substituted dihydropyridines, J.Chem. Cryst. 2000, 30, 415-422,
- Bosch Hubrecht R.W. J., Bogaerts W.F., B Syrett.C., Electrochemical Frequency Modulation: A New Electrochemical Technique for Online Corrosion Monitoring, Corros. Sci., 2001, 57 ,60-70.
- Fouda A. S., Abdallah Y. M., Nabil D., Dimethyl pyrimidine Derivative as Corrosion drugs for Carbon Steel in Hydrochloric Acid solutions, IJIRSET, 2014, 3,12965-12982.
- Yelri Y., Enriadi, Jamarun N., Gunawarman, Corrosion Inhibition Efficiency of mild Steel in Hydrochloric Acid by Adding theobroma Cacao Feel Extract, BCES, 2014, 14, 15-19.
- Otmocic Curkovic H., Marusic K., Stupnisek-Lisac E., and Telegdi J., Electrchemical and AFM study of Corrosion Iinhibition with Respect to Application Method, Chem. Biochem. Eng. Q. , 2009, 23 (1) 61- 66.
- Pralhibha S. B., Kotteeswaran P., Bheema Raju V., Study on the inhibition of MU steel Corrosion by Cationic Surfactant in HCl Medium, IOSR Journal of Applied Chemistry (IOSRJAC), 2012, 2, 45-53.
PDF Version of the article |
Flash Version of the article |
|
| [qrcode] | ||