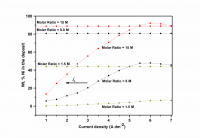
The Ni-Cd alloy coating was electrodeposited on mild steel (MS) from acid chloride bath using gelatin and glycerol as additives, individually and in combination. The bath composition and operating parameters have been optimized by conventional Hull cell method. The effect of current density (c.d.) on Ni content of the alloy was studied at different molar ratio of metal ions in the bath. The effects of c.d. and temperature on thickness, hardness, and composition and corrosion rate (CR) of the coatings were studied. Cyclic voltammetry (CV) study showed that (gelatin + glycerol) has significant effect on process of deposition and (gelatin + glycerol) worked synergistically to increase the Ni content by their preferential deposition and by suppressing the deposition of more readily depositable Cd2+ ions. Ni-Cd bath having both [Ni2+]/[Cd2+] = 1.5 and 8.0 exhibited anomalous type of codeposition at all c.d.’s studied. Corrosion behavior of the coatings evaluated by electrochemical methods demonstrated that the coating from bath [Ni2+]/[Cd2+] = 15, deposited at 4.0 A dm-2 is the most corrosion resistant. The superior corrosion resistance of Ni-Cd coatings at optimal c.d. was attributed to specific Ni (111), Ni (200), Cd (200) and Ni-Cd (862) reflections, evidenced by XRD study. The surface morphology was analyzed using SEM study, and results are discussed.
