Tag Archives: adsorption
New generation of acid Zn-Ni electrolyte for barrel application (Part 2)
The demand for Zinc Nickel coatings continuously increases in the automotive industry. Especially interesting are zinc nickel alloys with a nickel incorporation of 12–16 %, due to their high corrosion protection as well as superior wear and heat resistance as compared to pure zinc and other zinc alloy coatings.
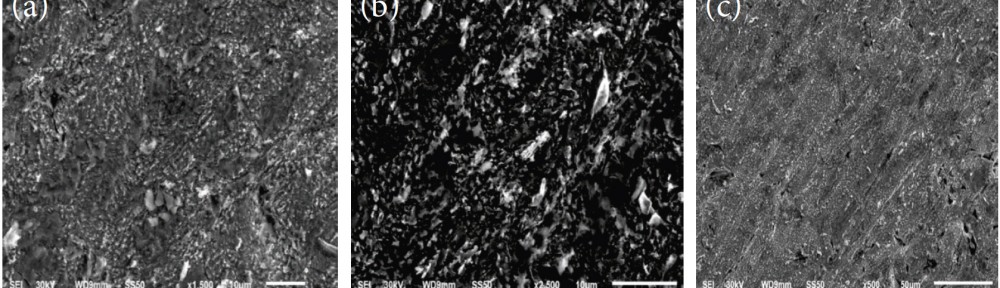
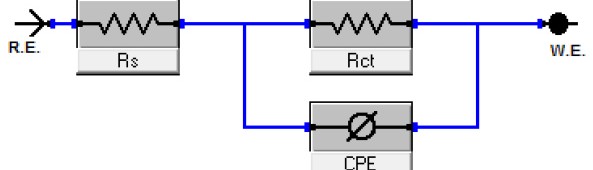
Despite many advantages of acid Zn-Ni electrolytes there are still some areas of application, like barrel plating or plating of complex-shaped parts, believed to be reserved for alkaline processes. In this paper zinc nickel coatings deposited from ammonium and boric acid-free acid zinc nickel electrolytes, with improved throwing power for rack and barrel applications are investigated. Their corrosion resistance, ductility and hardness will be presented. Moreover, their texture and morphology will be investigated using SEM, XRD and FIB methods. In the end thickness distribution and Ni-incorporation will be presented and compared to alkaline systems.
Adsorption and inhibitive properties of aqueous extracts of rosmarinus as a green corrosion extract for copper in HNO3
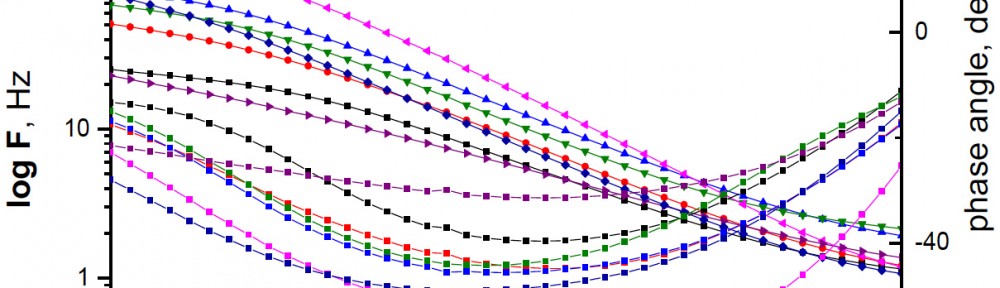
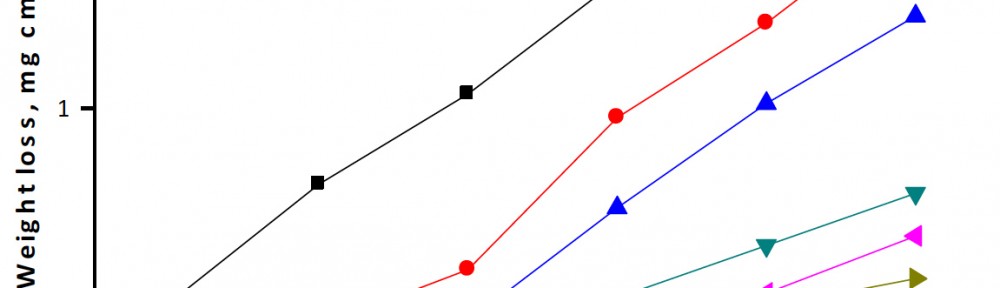
The efficiency of plant extract as corrosion extract for copper in 1M HNO3 medium was carried out using weight loss, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. The results showed variation in inhibition performance of the extract with varying concentration, immersion time and temperature. Langmuir isotherm was tested to describe the adsorption behavior of the extract on the copper surface. Potentiodynamic polarization study clearly revealed that this extract acts as a mixed type inhibitor i.e. the addition of the extract enhances both cathodic and anodic reactions. The results of the electrochemical impedance study showed a decrease in double layer capacitance and an increase in the charge transfer resistance. The results showed that rosmarinus extract could play significant role as corrosion inhibitor for copper in 1M HMO3.
Non ionic surfactants derived from phenol compounds as inhibitors for corrosion of aluminum in hydrochloric acid solution
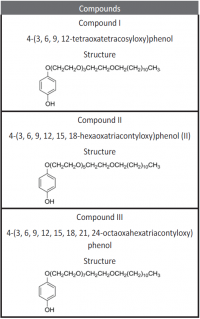
Inhibition of aluminum corrosion in 1M HCl in absence and presence of three compounds of non ionic surfactants compounds derived from phenol was investigated using hydrogen evolution reaction, weight loss galvanostatic polarization and electrochemical impedance spectroscopy techniques. It was found that the percentage inhibition increases with increasing the concentration of inhibitor, amount of ethylene oxide unit and with decreasing temperature. The inhibitive action of non ionic surfactant compounds was explained in terms of blocking the electrode surface by adsorption process. The adsorption process follows Langmuir isotherm. The polarization measurements showed that these inhibitors are acting as mixed inhibitors for both cathodic and anodic reaction. Electrochemical impedance spectroscopy technique exhibit one capacitive loop indicating that, the corrosion reaction is controlled by charge transfer process. Some activated thermodynamic parameters are calculated and explained.
Coumarin Derivatives as Corrosion Inhibitors for Zinc in HCl Solutions
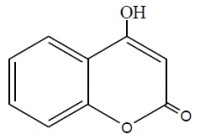
The inhibiting effect of some coumarin derivatives toward the corrosion of Zinc in 0.1M HCl solution was studied using weight loss and galvanostatic polarization techniques. Addition of KI to acidic medium containing the coumarin derivatives increases the inhibition efficiency of the system. The obtained results showed that the inhibition efficiency of these compounds increased by increasing their concentrations and decreased by rising the temperature, so that the adsorption of these compounds is physically adsorbed on the zinc surface. Temkin’s adsorption isotherm fits the experimental data for the studied compounds. Some thermodynamic parameters for the adsorption and activation process were computed. The values of Tafel slopes indicate that these compounds act as a mixed type inhibitors but cathode is more polarized when an external current was applied. The inhibitors are explained in terms of adsorption on the zinc surface. The order of inhibition efficiency are interpreted on the basis of the molecular structure, the subsistent groups and their charge densities of the coumarin derivatives.