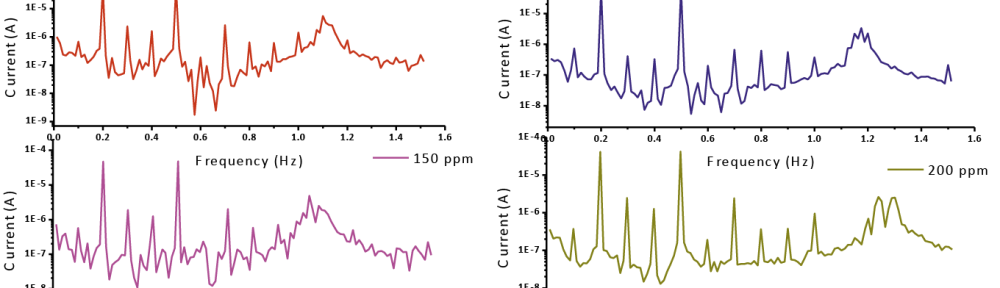Tag Archives: SEM-EDX
New generation of acid Zn-Ni electrolyte for barrel application (Part 2)
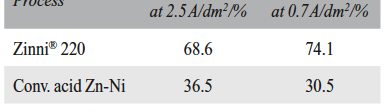
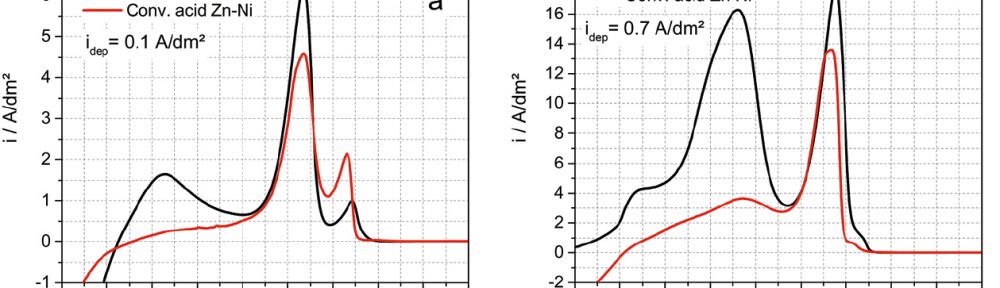
The demand for Zinc Nickel coatings continuously increases in the automotive industry. Especially interesting are zinc nickel alloys with a nickel incorporation of 12–16 %, due to their high corrosion protection as well as superior wear and heat resistance as compared to pure zinc and other zinc alloy coatings.
Despite many advantages of acid Zn-Ni electrolytes there are still some areas of application, like barrel plating or plating of complex-shaped parts, believed to be reserved for alkaline processes. In this paper zinc nickel coatings deposited from ammonium and boric acid-free acid zinc nickel electrolytes, with improved throwing power for rack and barrel applications are investigated. Their corrosion resistance, ductility and hardness will be presented. Moreover, their texture and morphology will be investigated using SEM, XRD and FIB methods. In the end thickness distribution and Ni-incorporation will be presented and compared to alkaline systems.
Electrochemical Investigations for the Corrosion Control of Aluminum using an Eco friendly Natural Inhibitor
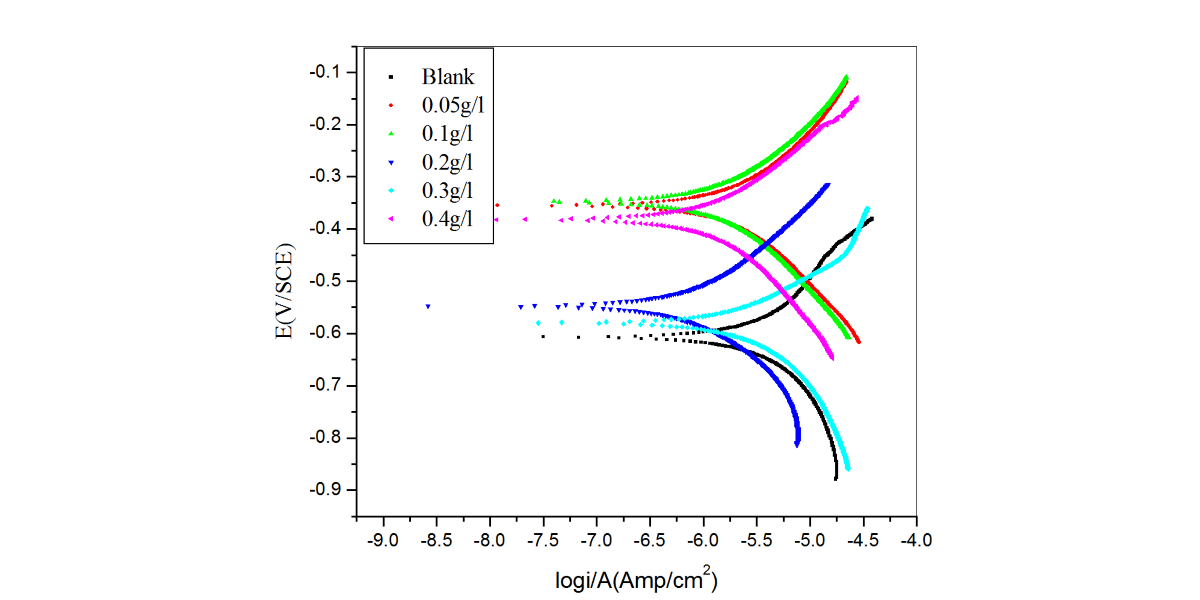
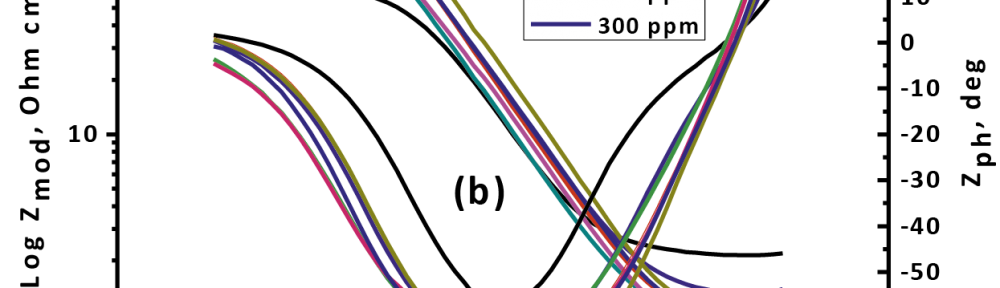
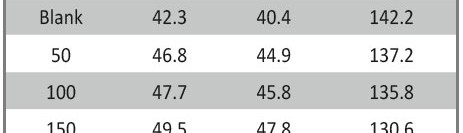
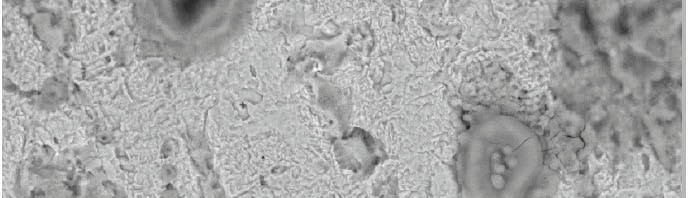
The inhibitive effect of Curry leaves extract (CLE) on the corrosion behavior of aluminum in sulfuric acid (pH = 3) was investigated by potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques in the temperature range of 30 °C to 50 °C. The study was done by varying the concentrations of inhibitor from 0.05 g L−1 to 0.4 g L−1. The surface morphology was studied using scanning electron microscopy (SEM) and Energy-dispersive X-ray spectroscopy (EDX). Inhibition efficiency was found to increase with increase in inhibitor concentration and decrease with increase in temperature. CLE acted as an anodic type inhibitor at lower concentrations of inhibitor and behaved as a mixed type at higher concentrations of inhibitor and underwent both physisorption and chemisorption on the surface of the metal and followed the Langmuir adsorption isotherm.