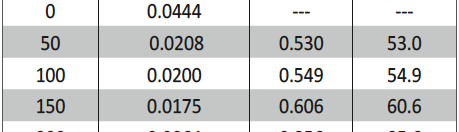
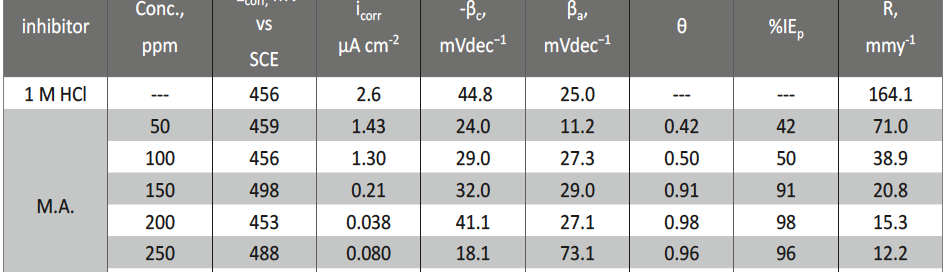
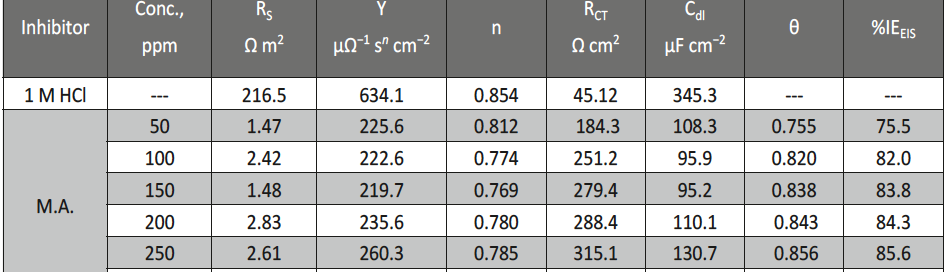
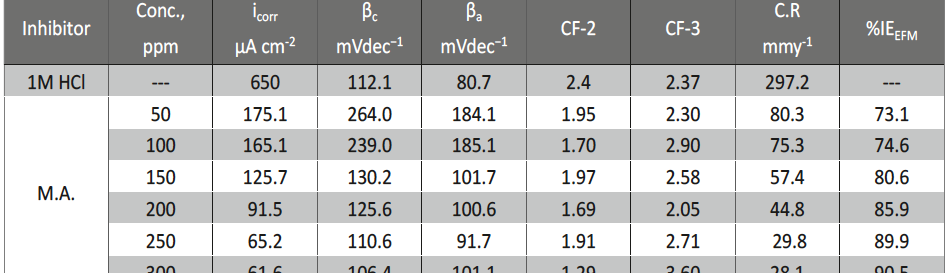
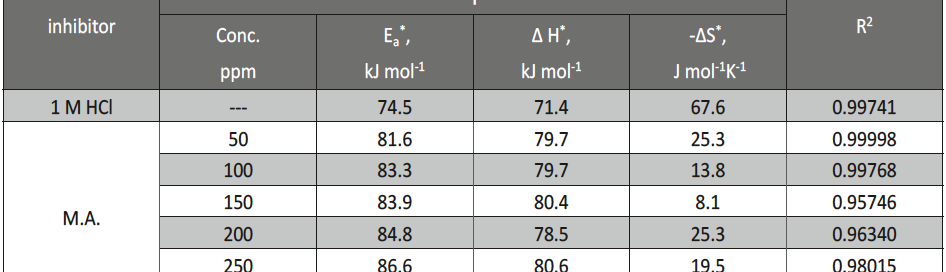
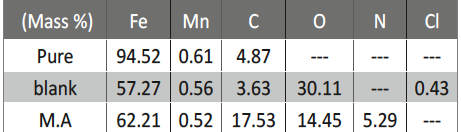
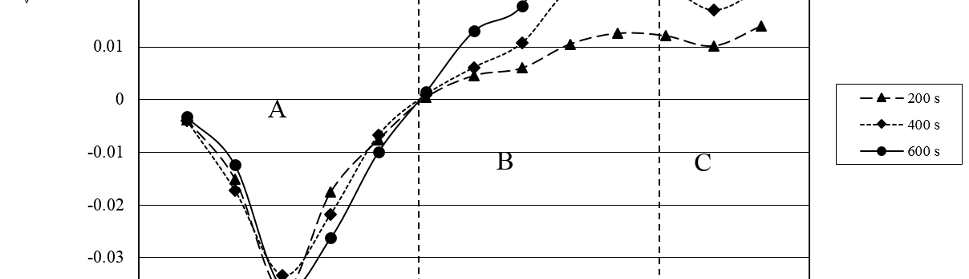
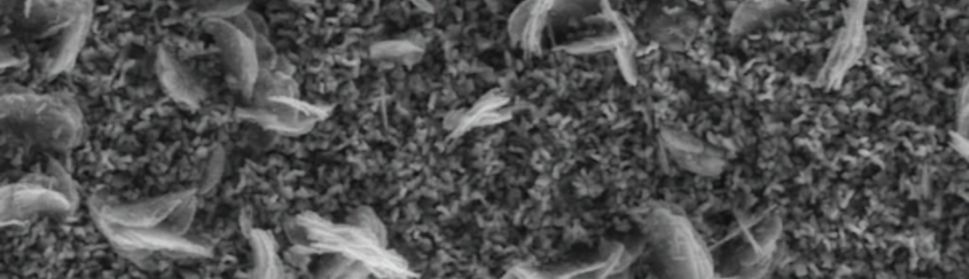
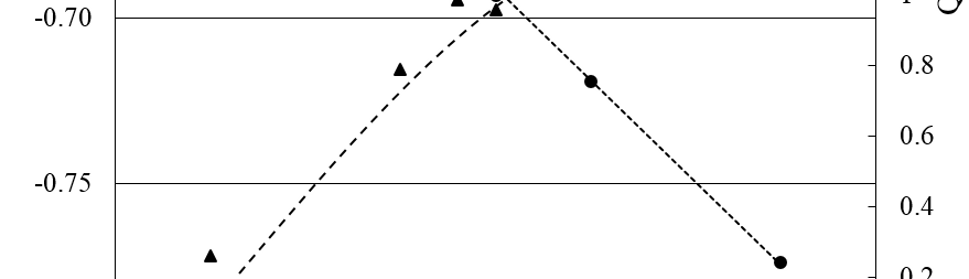
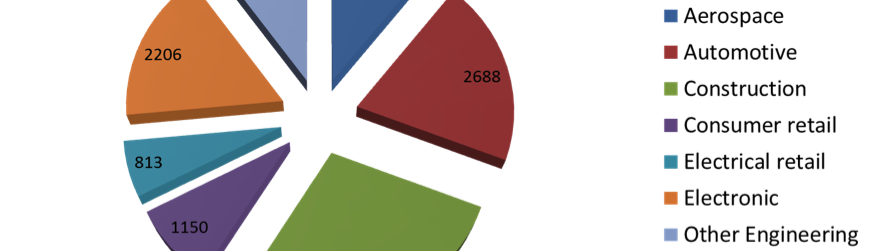The protection effect of malonic acid on carbon steel corrosion was studied in aerated stagnant 1M HCl solutions at 250C. Measurements were conducted under different experimental conditions using weight loss, Tafel polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. malonic acid was found to be good inhibitor of carbon steel corrosion in1 M HCl. The adsorption of this inhibitor is found to obey the Langmuir adsorption isotherm. The calculated activation energies proposed that the inhibitor molecules being physically adsorbed onto the metal surface. Polarization data revealed that this compound behave as mixed type inhibitor.
Category Archives: General
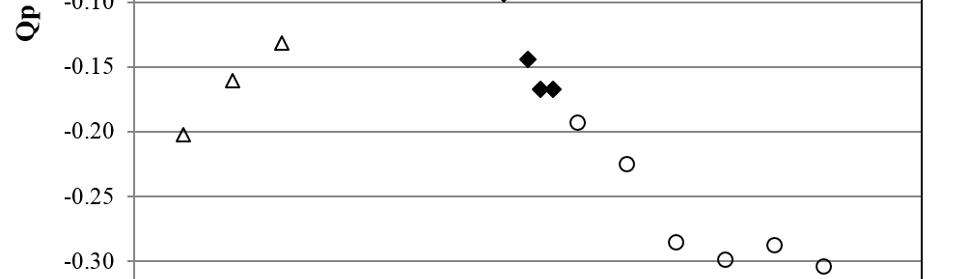
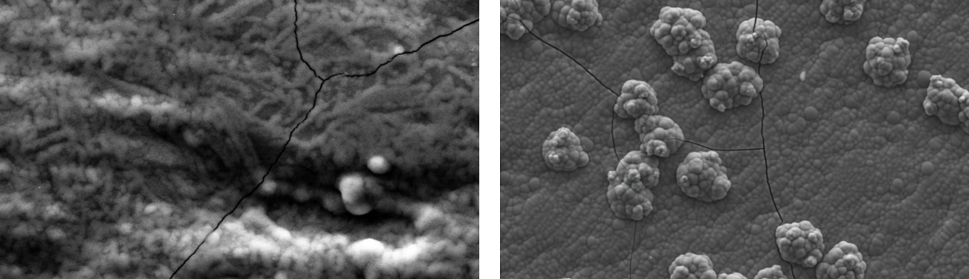
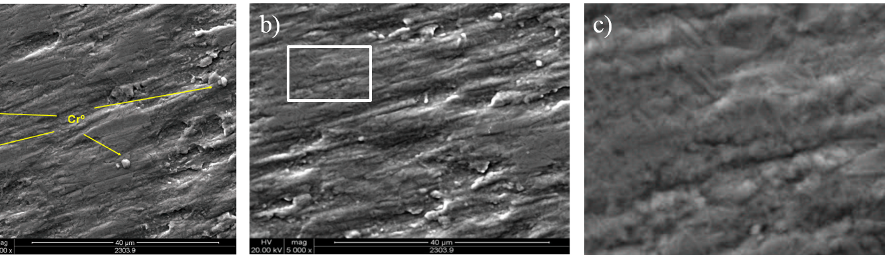
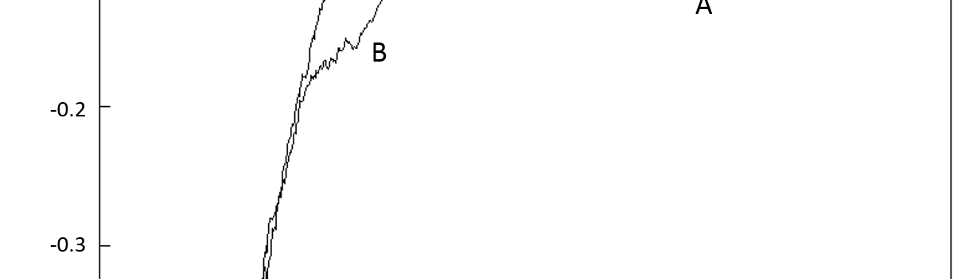
Square Wave Pulsating Overpotential Treatment of Chromic Acid Solution
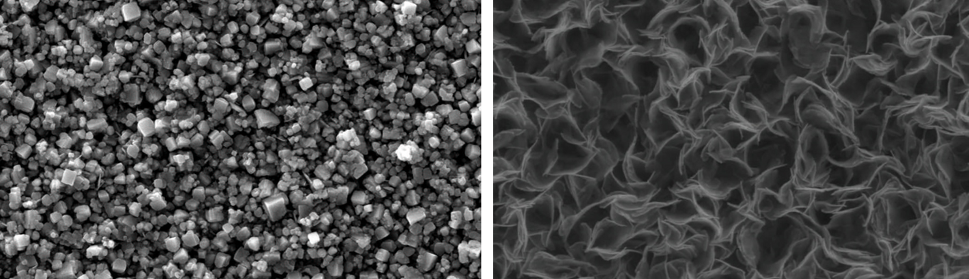
Systematic investigation of square wave pulsating overpotential (SWPO) treatment of a chromic acid solution is presented. Some preliminary potentiodynamic scans, potentiostatic deposits, potential steps and capacitance vs. potential curves were measured in order to establish the practical range of the SWPO signal parameters. The results show that properly adjusting the electrochemical parameters of the cyclic alternating potential perturbation it is possible to obtain cracked or crack free metallic chromium or chromium oxide/hydroxide mixed coatings. The different deposit morphologies were characterized through scanning electron microscopy and their chemical composition and micro hardness was measured. Some insight into the possible mechanisms of coating growth under this potential cycling treatment is given.
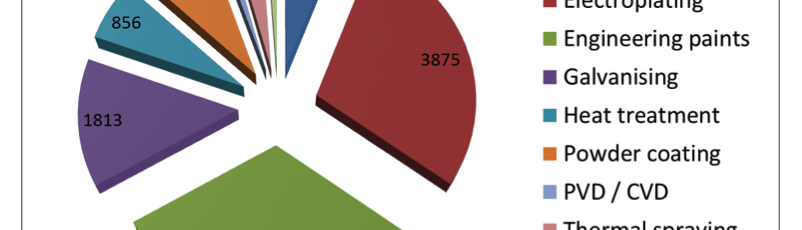
Overview of the UK Surface engineering sector
Surface engineering is a sub-discipline of materials science and covers a multitude of processes such as electroplating, anodising, electro-polishing, heat treatment processes, physical vapour deposition and the like. The term surface engineering was promoted by, amongst others, Professor Tom Bell of the University of Birmingham defining it as “the design of surface and substrate together as a functionally graded system to give a cost-effective performance enhancement of which neither is capable on its own”. Imagine a world without cars, aeroplanes, trains, computers, mobile phones, medical implants, buildings, electronics, in fact virtually no manufactured products – that’s a world without surface engineering. Thus, the application of surface engineering is vital to the success of almost every commercial and industrial product: from aero engines to aeroplanes, from iPods to surgical implants and from razor blades to racing cars.
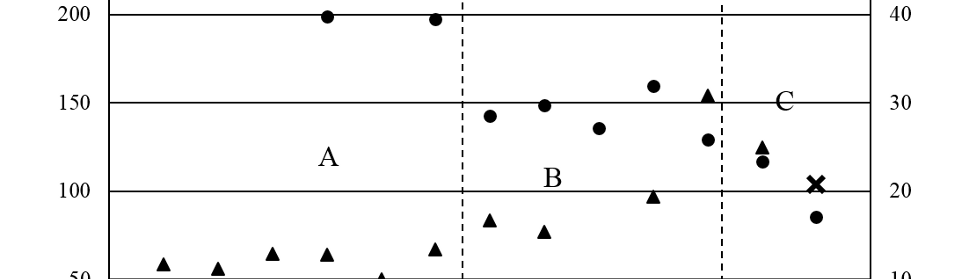
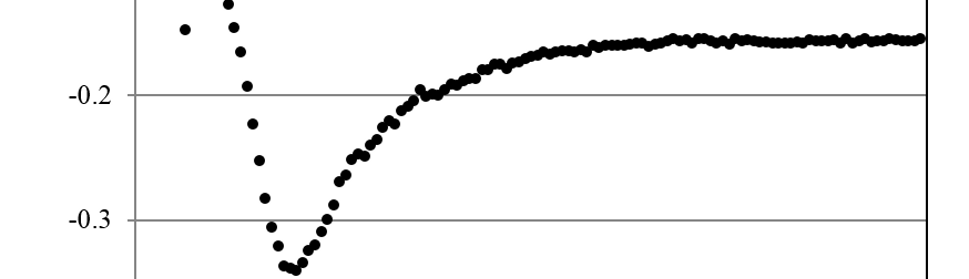
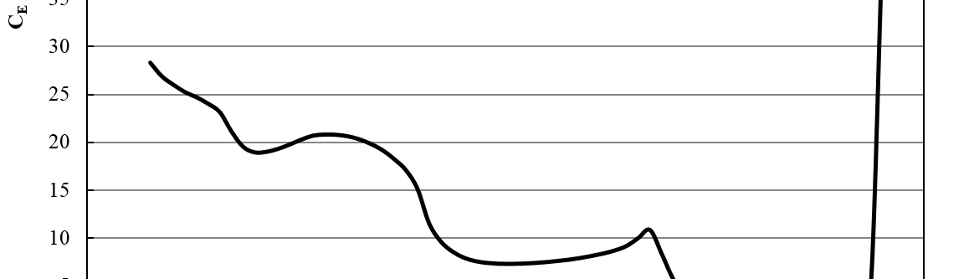
Adsorption and Corrosion Inhibition Characteristics of Some Thiophene-3-Carbohydrazide Derivatives on Low Carbon Steel in Hydrochloric Acid Solutions
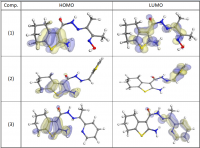
New compounds of corrosion inhibitors namely amino-N’-(3-(hydroxyimino)butan-2-ylidene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (1), amino-N’-(thiophen-2-ylmethylene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (2) and amino-N’-(1-(pyridin-2-yl)ethylidene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (3) were synthesized and its inhibiting action on the corrosion of carbon steel in 1 M hydrochloric acid at 25ºC was investigated by various corrosion monitoring techniques. A Potentiodynamic polarization, AC impedance and electrochemical frequency modulation methods have been used. Potentiodynamic polarization studies showed that these derivatives were mixed type inhibitors. The effect of temperature on the corrosion behavior of carbon steel in 1 M HCl with the addition of these compounds were studied in the temperatures 25 and 45ºC. The adsorption of these inhibitors on carbon steel surface from hydrochloric acid obeyed the Langmuir adsorption isotherm. Quantum chemical method is used to explore the relationship between the inhibitors molecular properties and their inhibition efficiency.
Sodium-bismuth-lead low temperature liquid metal battery

The development of a low temperature liquid metal battery based on ionic liquids namely, sodium-bis(trifluoromethylsulfonyl)imide (Na[TFSI]) in tetraethylammonium-bis(trifluoromethylsulfonyl) imide ([TEA][TFSI]) will be discussed. Such a battery should be easily accessible for fluid flow measurements which is still a challenge with the conventional high temperature systems. Cells comprising a Na negative electrode, 20 mole% Na[TFSI] in [TEA][TFSI] ionic liquid electrolyte and a Pb-Bi eutectic positive electrode were constructed and operated at 160 °C. Galvanostatic cycling experiments were conducted at low C rates (C/26) for 13 h corresponding to 50% depth of discharge. A discharge capacity of 565 mAh/g was found. Furthermore electrochemical impedance spectroscopy was used to characterize the aging of the cells.