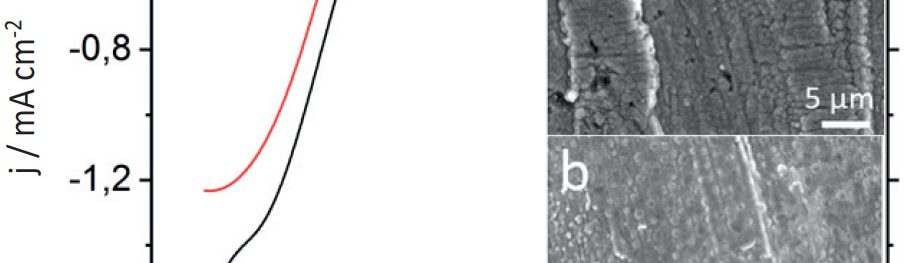
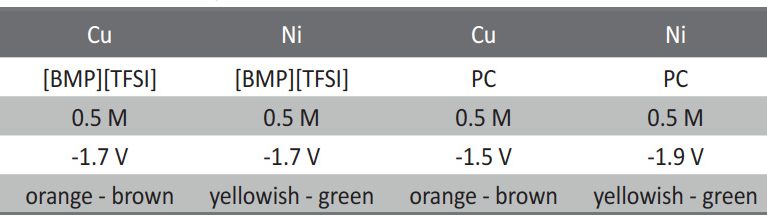
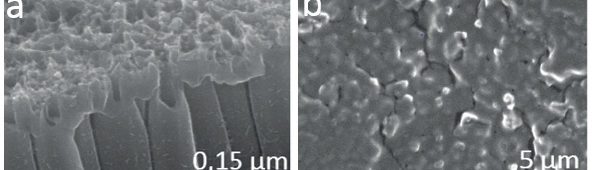
Electrochemical reduction of silicon from SiCl4 in 1-butyl-1-metyl-pyrrolidinium bis(trifluoromethylsulfonyl)imide [BMP][TFSI] and in propylene carbonate (PC) with SiCl4 as a precursor is performed at room temperature. The process is studied by means of Linear Sweep Voltammetry and chronoamperometry. The results exhibit considerable differences during the silicon deposition for copper and nickel. Scanning Electron Microscopy (SEM) of the layers shows a rough surface morphology. The composition of Si deposit is confirmed by Energy Dispersive X-ray analysis (EDX). Furthermore, the deposition of silicon onto TiO2 nanotubes is discussed. In conclusion, a method of recycling the used ionic liquid by a simple extraction procedure is presented.
Tag Archives: Ionic liquids
Sodium-bismuth-lead low temperature liquid metal battery

The development of a low temperature liquid metal battery based on ionic liquids namely, sodium-bis(trifluoromethylsulfonyl)imide (Na[TFSI]) in tetraethylammonium-bis(trifluoromethylsulfonyl) imide ([TEA][TFSI]) will be discussed. Such a battery should be easily accessible for fluid flow measurements which is still a challenge with the conventional high temperature systems. Cells comprising a Na negative electrode, 20 mole% Na[TFSI] in [TEA][TFSI] ionic liquid electrolyte and a Pb-Bi eutectic positive electrode were constructed and operated at 160 °C. Galvanostatic cycling experiments were conducted at low C rates (C/26) for 13 h corresponding to 50% depth of discharge. A discharge capacity of 565 mAh/g was found. Furthermore electrochemical impedance spectroscopy was used to characterize the aging of the cells.