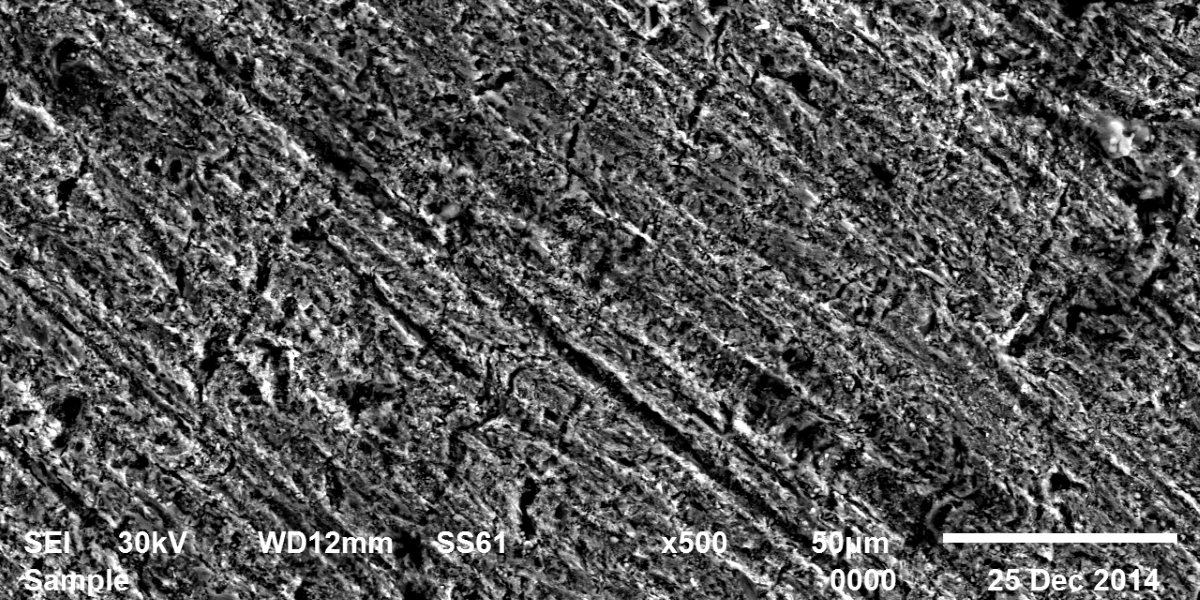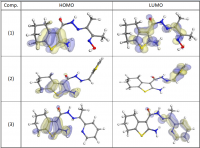
New compounds of corrosion inhibitors namely amino-N’-(3-(hydroxyimino)butan-2-ylidene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (1), amino-N’-(thiophen-2-ylmethylene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (2) and amino-N’-(1-(pyridin-2-yl)ethylidene)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carbohydrazide (3) were synthesized and its inhibiting action on the corrosion of carbon steel in 1 M hydrochloric acid at 25ºC was investigated by various corrosion monitoring techniques. A Potentiodynamic polarization, AC impedance and electrochemical frequency modulation methods have been used. Potentiodynamic polarization studies showed that these derivatives were mixed type inhibitors. The effect of temperature on the corrosion behavior of carbon steel in 1 M HCl with the addition of these compounds were studied in the temperatures 25 and 45ºC. The adsorption of these inhibitors on carbon steel surface from hydrochloric acid obeyed the Langmuir adsorption isotherm. Quantum chemical method is used to explore the relationship between the inhibitors molecular properties and their inhibition efficiency.