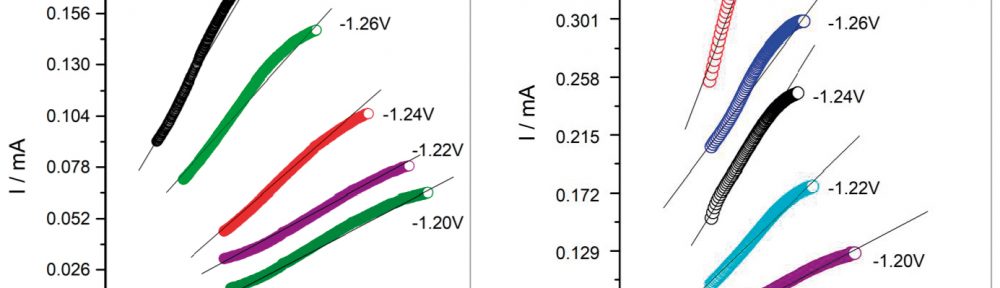
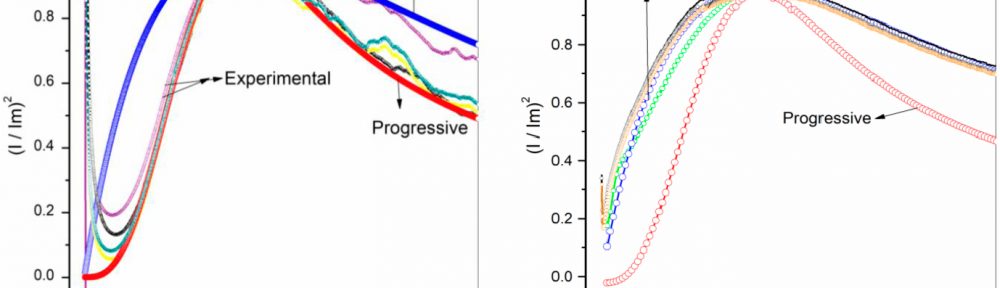
In present investigation, a new brightener was synthesized by condensation of 3, 4, 5-Trimethoxy benzaldehyde and Glycine (TG). Hull cell experiments were conducted to optimize the plating bath components and operating parameters. To examine the influence of TG on nucleation mechanism of Zn-Co alloy electrodeposition, cyclic voltammetry and chronoamperometry study was carried out. Schariffker and Hills model was used to analyze current transients, which in presence of TG confirmed instantaneous nucleation. Corrosion studies were done using potentiodynamic polarization and electrochemical impedance spectroscopic technique, in 3.5 wt. % NaCl for bright and dull zinc-cobalt alloy coatings. Phase structure, surface morphology and brightness of the deposit were characterized by X-ray diffraction analysis, scanning electron microscopy and reflectance studies. These studies revealed the role of TG in modifying the nucleation mechanism and surface morphology of zinc-cobalt alloy crystallites and thereby producing a bright corrosion resistant Zn-Co alloy coating on mild steel substrate.
Tag Archives: nucleation
Electrochemical Nucleation and Growth of Gold on Embedded Rhenium Nanowires
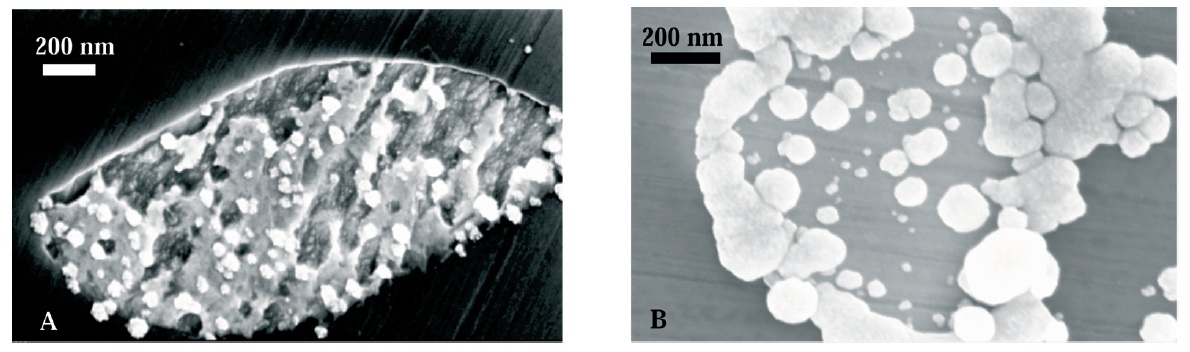
The formation of gold nanoelectrode arrays was investigated by electrodeposition of the metal along the pores left on directionally solidified NiAl-Re eutectics by selective dissolution of the rhenium fibre. After the necessary pre-treatment for the passivation of the NiAl matrix and dissolution of the rhenium fibres to create arrays of nanopores (diameter ~ 400 nm), the electrodeposition of gold into the pores was initially investigated by examining the growth of the deposits with the application of cathodic pulses. It was observed that the size of the gold deposits increased with the duration of the applied cathodic pulse once an initial charge of ~ 800 C/m2 was overcome. The necessity of applying charges larger than that to observe significant deposits is due to the occurrence of a series of processes alongside the electrodeposition: charging of the oxides present on the eutectic and reduction of any remaining rhenium oxide on the rhenium fibres. Electrodeposition under potentiostatic conditions yielded a better control over the obtained gold structures, and enabled the selective filling of the pores. However, the recorded current transients under those experimental conditions did not obey any of the proposed models for nucleation and growth accurately. This was explained by the simultaneous formation of rhenium oxides and the interference of this process on the recorded current. Nevertheless, the studies reported give initial information on the electrochemical processes that take place when complex metallic substrates are employed for electrodeposition.