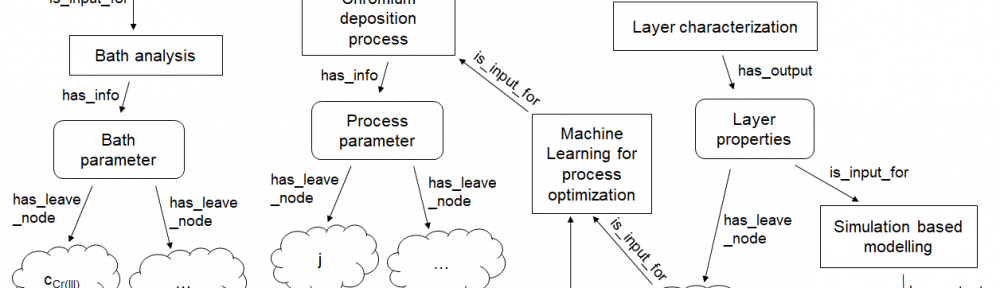
In order to make material design processes more efficient in the future, the underlying multidimensional process parameter spaces must be systematically explored using digitalisation techniques such as machine learning (ML) and digital simulation. In this paper we shortly review essential concepts for the digitalisation of electrodeposition processes with a special focus on chromium plating from trivalent electrolytes.
Author Archives: Prof. Dr. rer. nat. habil. Andreas Bund
Electrochemical deposition of silicon from organic electrolytes
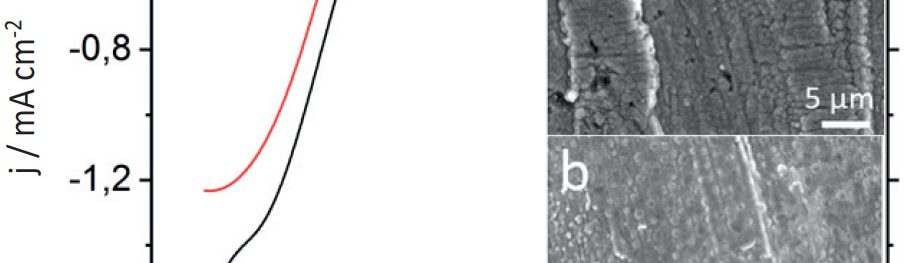
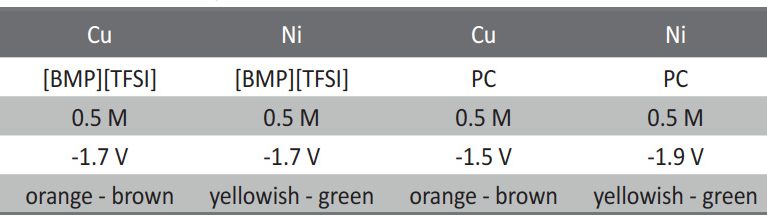
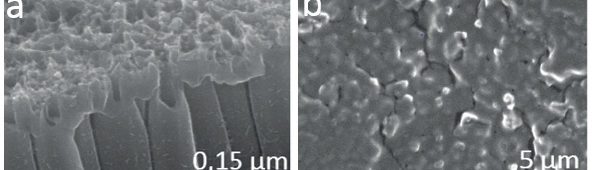
Electrochemical reduction of silicon from SiCl4 in 1-butyl-1-metyl-pyrrolidinium bis(trifluoromethylsulfonyl)imide [BMP][TFSI] and in propylene carbonate (PC) with SiCl4 as a precursor is performed at room temperature. The process is studied by means of Linear Sweep Voltammetry and chronoamperometry. The results exhibit considerable differences during the silicon deposition for copper and nickel. Scanning Electron Microscopy (SEM) of the layers shows a rough surface morphology. The composition of Si deposit is confirmed by Energy Dispersive X-ray analysis (EDX). Furthermore, the deposition of silicon onto TiO2 nanotubes is discussed. In conclusion, a method of recycling the used ionic liquid by a simple extraction procedure is presented.
Characterization and influence on the fatigue properties of the metal-turn-over of an electroless nickel coating on an AlCuMgFeNi alloy
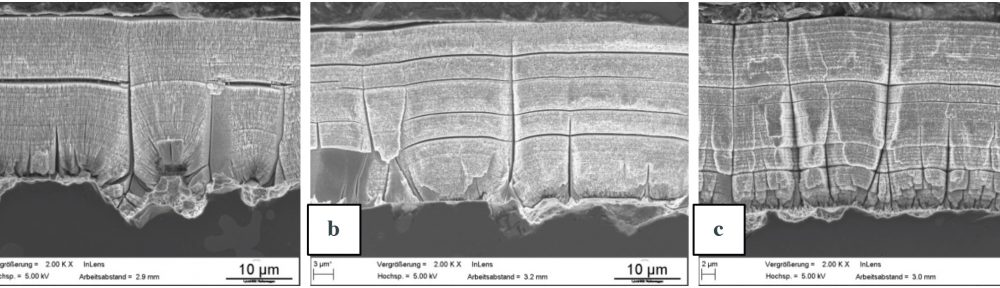
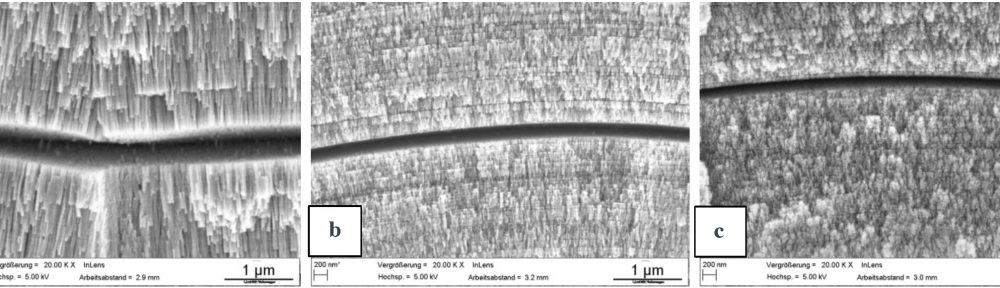
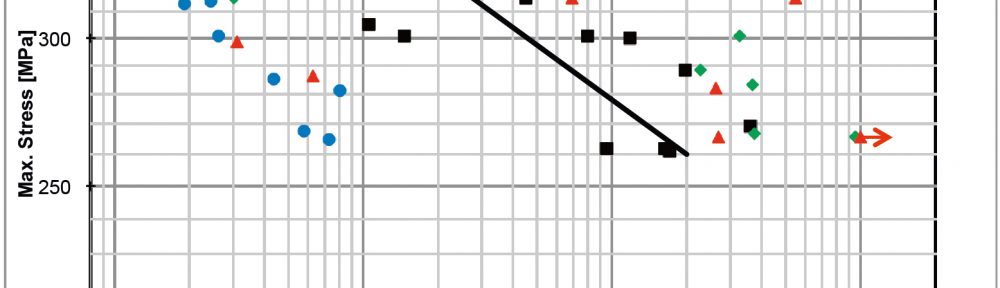
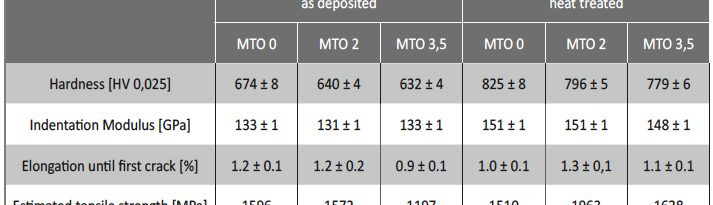
In this paper the influence of a mid-phosphorous electroless nickel coating on EN-AW 2618A was studied. Special emphasis was put on the metalturn-over (MTO) and a heat treatment on the coating properties and their influence on the fatigue properties. The increasing MTO leads to an increase in phosphorous content resulting in a reduction of hardness, while the ductility is much less affected. The low temperature heat treatment increases the hardness through a crystal growth. The fatigue tests show, that the electroless nickel coating can both have a positive as well as a negative influence on the fatigue properties. At higher mechanical stresses the deposit tends to reduce the lifetime, while at lower loads the lifetime gets increased. The reduction of lifetime is caused by defects in the coating which act as stress concentrators. An increase in MTO leads to a higher amount of coating defects and therefore a higher possibility for a reduction of the lifetime. Further research has to focus on the growth mechanisms of those defects since their influence seems to be more significant than other factors like the phosphorous content.
German-Korean Workshop: “Novel electrochemical technologies in material science for applications in information and energy technology (NEAT)”
The German-Korean Workshop „Novel electrochemical technologies in material science for applications in information and energy technology“ (NEAT) took place between 24th and 26th of September at the Jagd- und Berghotel Gabelbach in Ilmenau, Germany. The workshop was organized by the Electrochemistry and Electroplating Group (TU Ilmenau, FG ECG, Prof Andreas Bund) and the Korea Institute of Materials Sciene (KIMS, Dr. Kyu-Hwan Lee). The ECG group of TU Ilmenau is for many years in contact with several research centres in Korea such as Hanyang University (Seoul), KIMS (Korea Institute of Materials Science, Changwon) or the PCB Research Center of Korea Polytechnic University (Siheung). This collaboration and the workshop were funded by the German Ministry of Education and Research BMBF (via the German National Aeronautics and Space Research Center, DLF, as project promoter) in order to advance the scientific and technical collaboration between both countries. The aim of the workshop was the preparation of joint projects in electroplating, electrocatalysis and energy storage. More than 20 scientists form Korea and Germany participated in this event, most of them young researchers. Continue reading…
Sodium-bismuth-lead low temperature liquid metal battery

The development of a low temperature liquid metal battery based on ionic liquids namely, sodium-bis(trifluoromethylsulfonyl)imide (Na[TFSI]) in tetraethylammonium-bis(trifluoromethylsulfonyl) imide ([TEA][TFSI]) will be discussed. Such a battery should be easily accessible for fluid flow measurements which is still a challenge with the conventional high temperature systems. Cells comprising a Na negative electrode, 20 mole% Na[TFSI] in [TEA][TFSI] ionic liquid electrolyte and a Pb-Bi eutectic positive electrode were constructed and operated at 160 °C. Galvanostatic cycling experiments were conducted at low C rates (C/26) for 13 h corresponding to 50% depth of discharge. A discharge capacity of 565 mAh/g was found. Furthermore electrochemical impedance spectroscopy was used to characterize the aging of the cells.