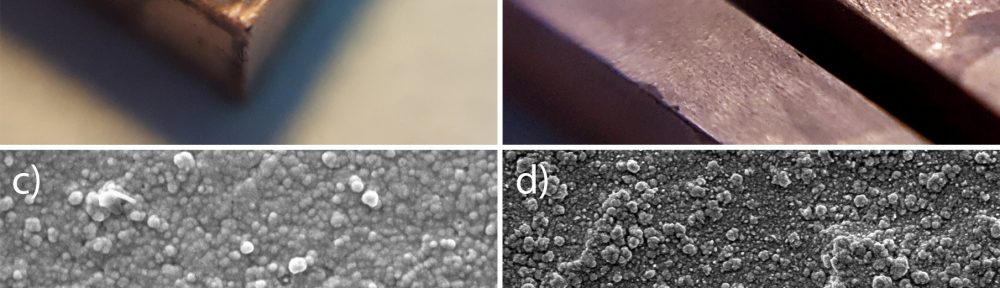
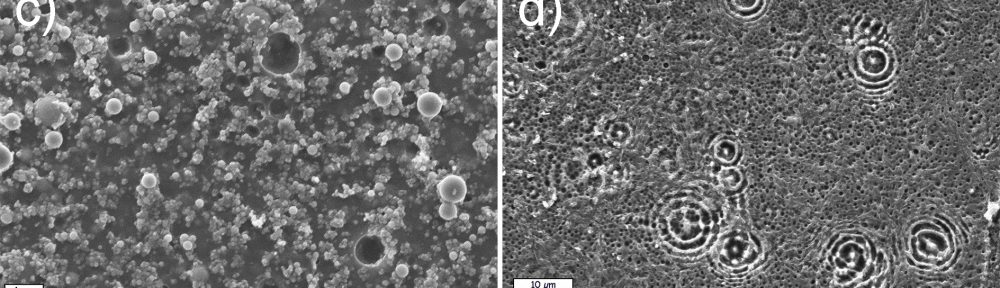
In the present paper, the influence of different wet pretreatment routes on the final quality of electroless layers plated on stereolithography resins is investigated. Two pretreatment methods, acidic and alkaline, are employed. Acidic etching is unable to provide acceptable results in terms of surface quality. On the contrary, alkaline etching guarantees a bright copper surface coupled with a level of adhesion high enough to pass a standard peel test. ATR FT-IR is employed to investigate the chemical reactions occurring on the resins during the pretreatment, evidencing a major role of the ester hydrolysis process on the depolymerization of the material and on the formation of new functional groups on the surface. Resin hydrolysis is linked with increase in surface roughness and wettability, parameters that strongly determine final metal adhesion. The combination of ATR FT-IT, contact angle and roughness measurement constitutes a possible combined methodology to follow the evolution of surface pretreatment on different stereolithography resins.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany