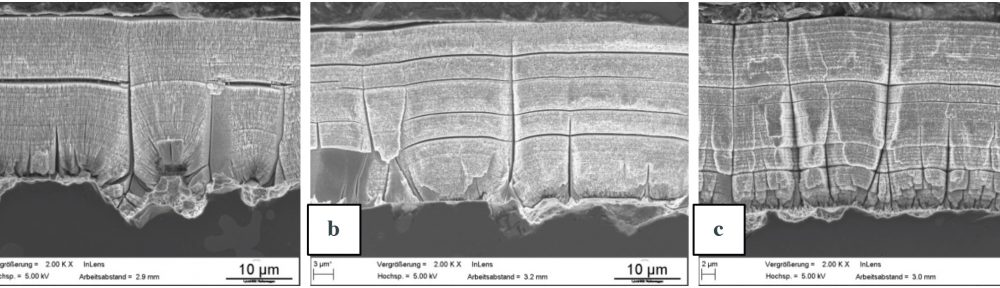
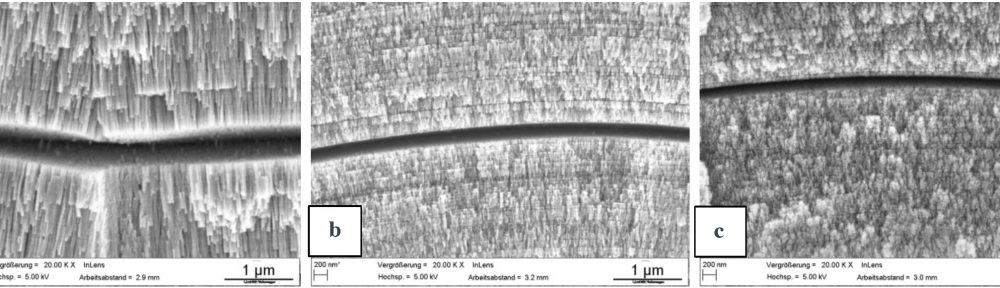
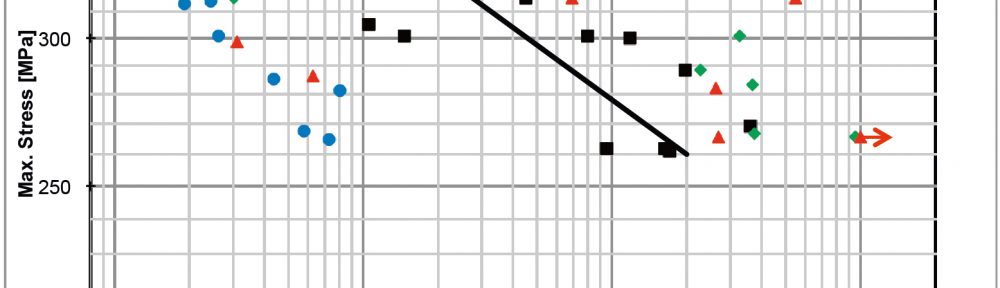
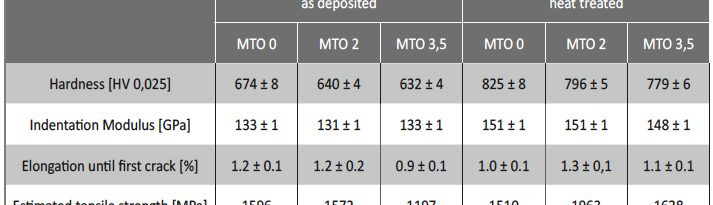
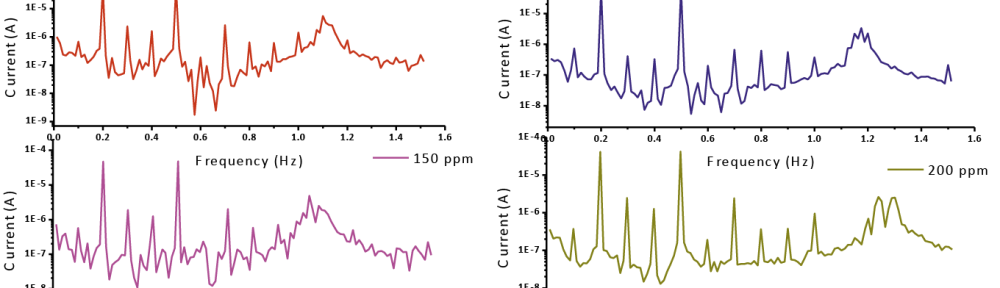In this paper the influence of a mid-phosphorous electroless nickel coating on EN-AW 2618A was studied. Special emphasis was put on the metalturn-over (MTO) and a heat treatment on the coating properties and their influence on the fatigue properties. The increasing MTO leads to an increase in phosphorous content resulting in a reduction of hardness, while the ductility is much less affected. The low temperature heat treatment increases the hardness through a crystal growth. The fatigue tests show, that the electroless nickel coating can both have a positive as well as a negative influence on the fatigue properties. At higher mechanical stresses the deposit tends to reduce the lifetime, while at lower loads the lifetime gets increased. The reduction of lifetime is caused by defects in the coating which act as stress concentrators. An increase in MTO leads to a higher amount of coating defects and therefore a higher possibility for a reduction of the lifetime. Further research has to focus on the growth mechanisms of those defects since their influence seems to be more significant than other factors like the phosphorous content.
Category Archives: General
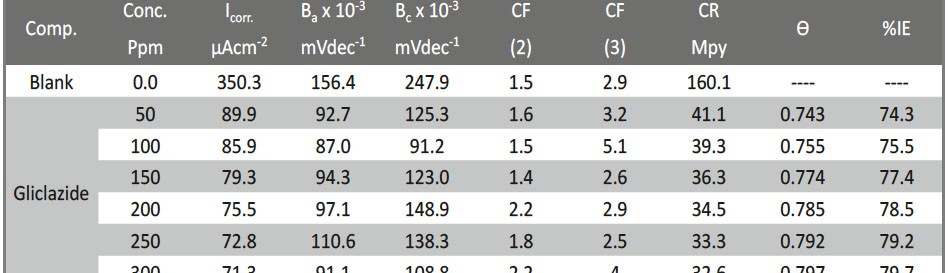
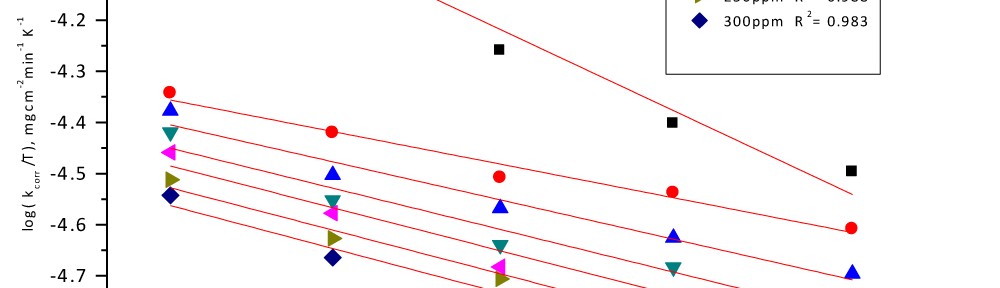
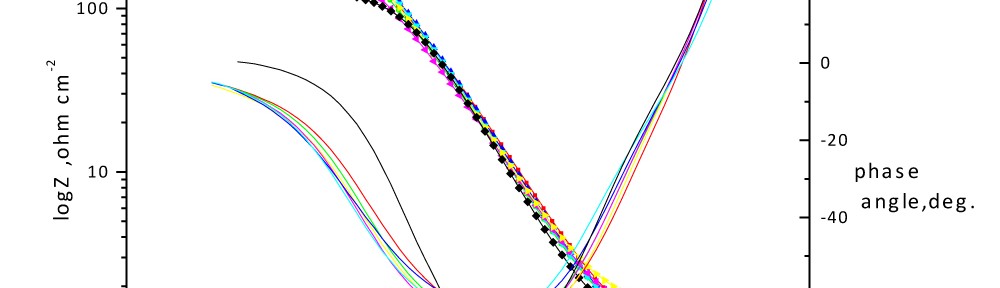
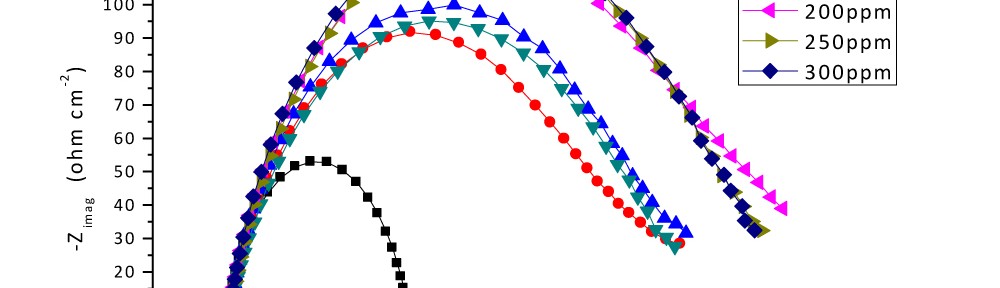
Corrosion Inhibition of Carbon Steel in hydrochloric acid medium using Gliclazide drug
Delonix Regia Leaf Extract as Environmental Friendly and Safe Corrosion Inhibitor for Carbon Steel in Aqueous Solutions
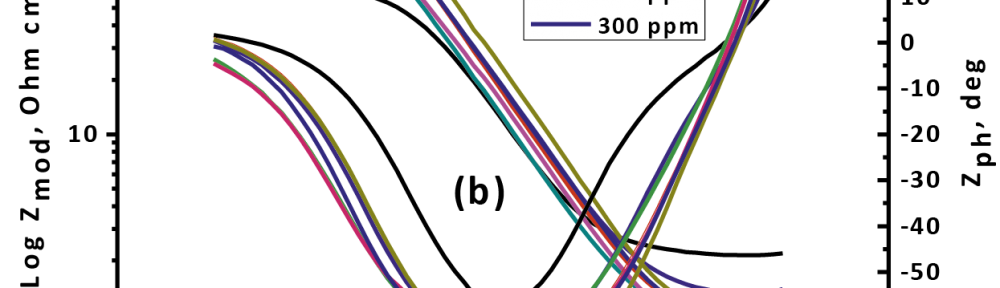
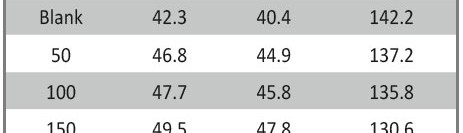
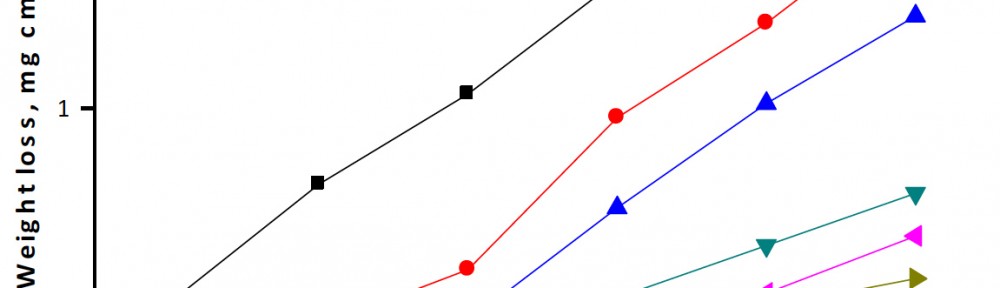
Delonix regia leaf extract activity as a green corrosion inhibitor (environmental friendly) for carbon steel (CS) in 1M HCl has been studied using weight loss (WL), potentiodynamic polarization (PP), electrochemical frequency modulation (EFM) and electrochemical impedance spectroscopy (EIS). The weight loss results show that Delonix regia leaf extract is an excellent corrosion inhibitor. The inhibition efficiency (IE) increases with temperature from 25 to 45oC, reaching a maximum value of 78.8 % at the highest concentration of 300 ppm at the temperature of 45oC. Polarization measurements demonstrate that the Delonix regia leaf extract acts as a mixed type inhibitor. Nyquist plot illustrates that on increasing Delonix regia leaf extract dose, the charge transfer increases and the double layer capacitance decreases. The adsorption of Delonix regia leaf extract on CS obeys Temkin adsorption isotherm.
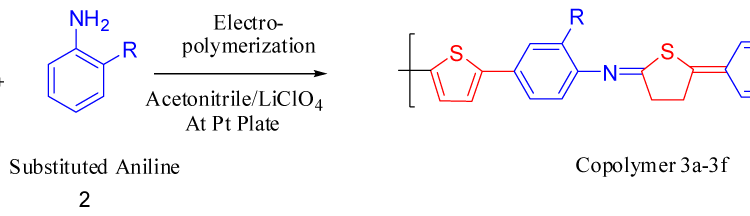
Electrochemical Deposition and Characterization of Conjugated Copolymers of Thiophene and Aniline
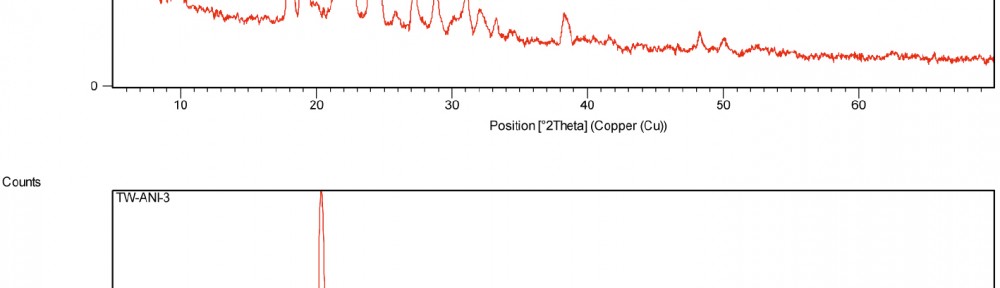
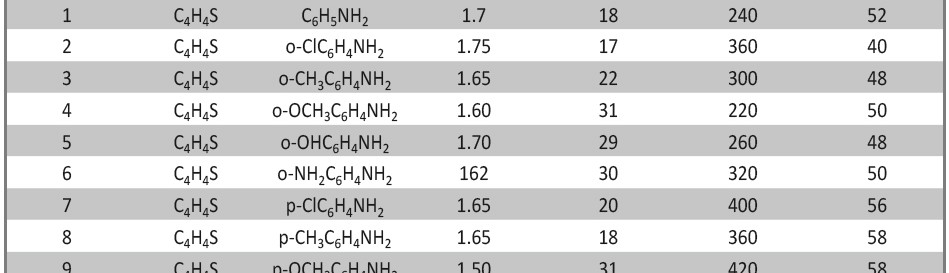
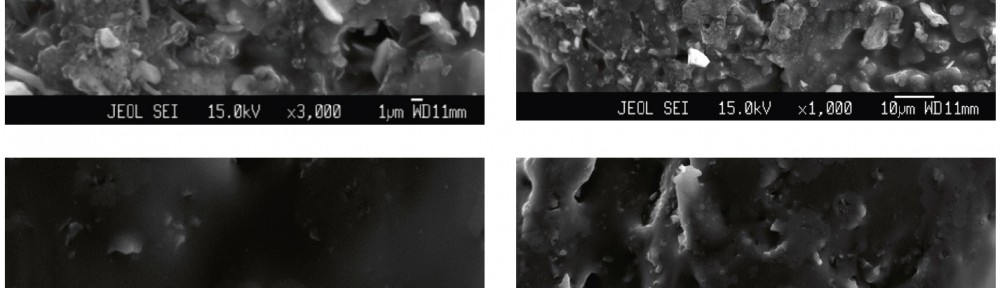
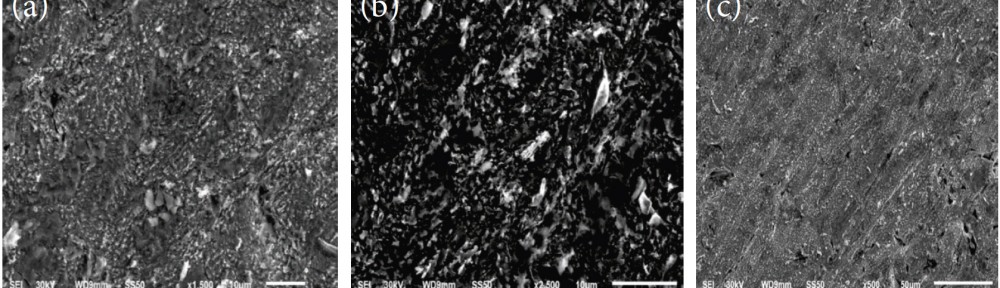
A new series of copolymers, obtained by reacting aniline as electron donor with thiophene as electron acceptor in a donor–acceptor structure (poly-thio-co-ani), were synthesized via electrochemical polymerization using acetonitrile as a solvent and lithium perchlorate as supporting electrolyte. The copolymer have better solubility in DMSO and KOH than the corresponding homopolymers. Copolymerization of aniline and thiophene was studied by UV-visible and FT-IR spectroscopy. In order to analyze their structure and characteristics X-ray diffraction analysis was applied and the samples were photographed under scanning electron microscope (SEM) for microstructure analysis and morphological property. Electrochemical properties were observed by cyclic voltammetry.
Adsorption and inhibitive properties of aqueous extracts of rosmarinus as a green corrosion extract for copper in HNO3
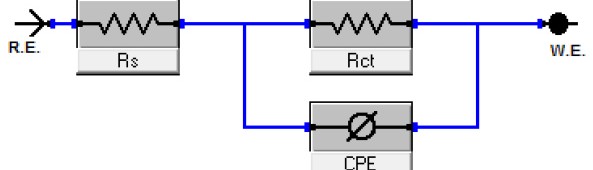
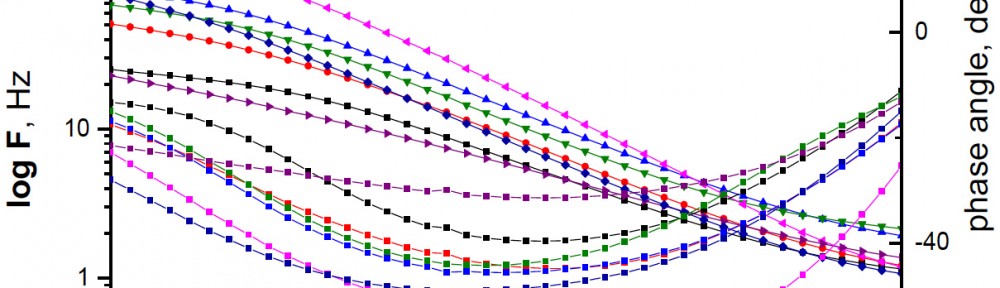
The efficiency of plant extract as corrosion extract for copper in 1M HNO3 medium was carried out using weight loss, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and electrochemical frequency modulation (EFM) techniques. The results showed variation in inhibition performance of the extract with varying concentration, immersion time and temperature. Langmuir isotherm was tested to describe the adsorption behavior of the extract on the copper surface. Potentiodynamic polarization study clearly revealed that this extract acts as a mixed type inhibitor i.e. the addition of the extract enhances both cathodic and anodic reactions. The results of the electrochemical impedance study showed a decrease in double layer capacitance and an increase in the charge transfer resistance. The results showed that rosmarinus extract could play significant role as corrosion inhibitor for copper in 1M HMO3.