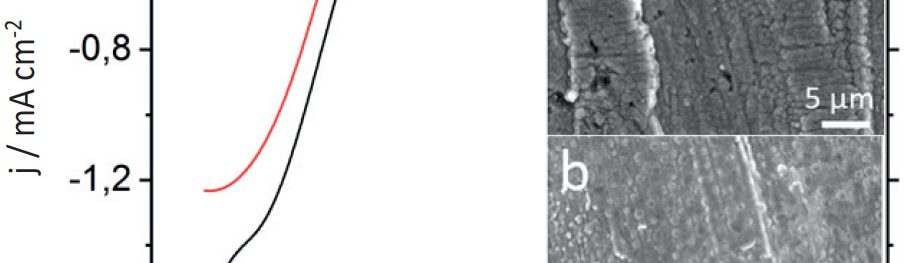
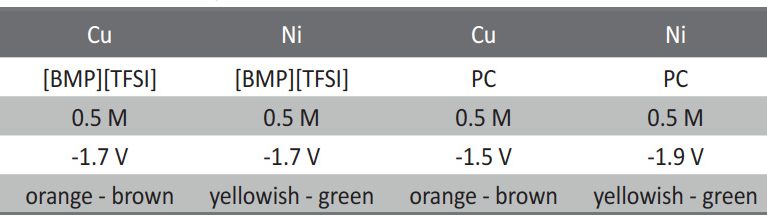
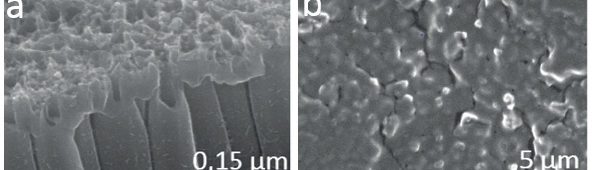
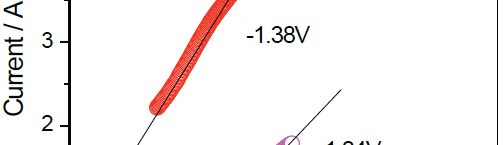
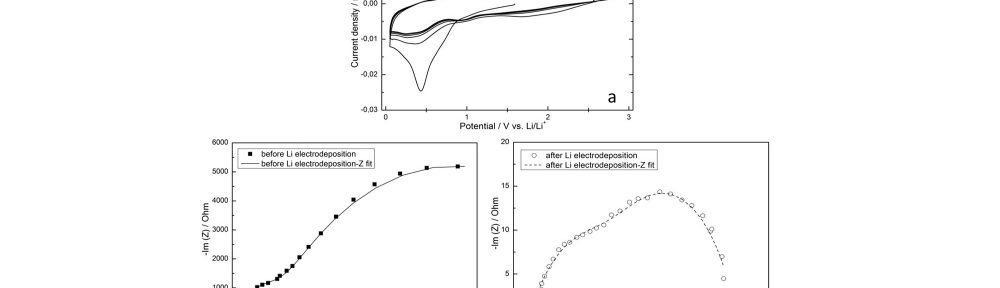
Electrochemical reduction of silicon from SiCl4 in 1-butyl-1-metyl-pyrrolidinium bis(trifluoromethylsulfonyl)imide [BMP][TFSI] and in propylene carbonate (PC) with SiCl4 as a precursor is performed at room temperature. The process is studied by means of Linear Sweep Voltammetry and chronoamperometry. The results exhibit considerable differences during the silicon deposition for copper and nickel. Scanning Electron Microscopy (SEM) of the layers shows a rough surface morphology. The composition of Si deposit is confirmed by Energy Dispersive X-ray analysis (EDX). Furthermore, the deposition of silicon onto TiO2 nanotubes is discussed. In conclusion, a method of recycling the used ionic liquid by a simple extraction procedure is presented.
Category Archives: General
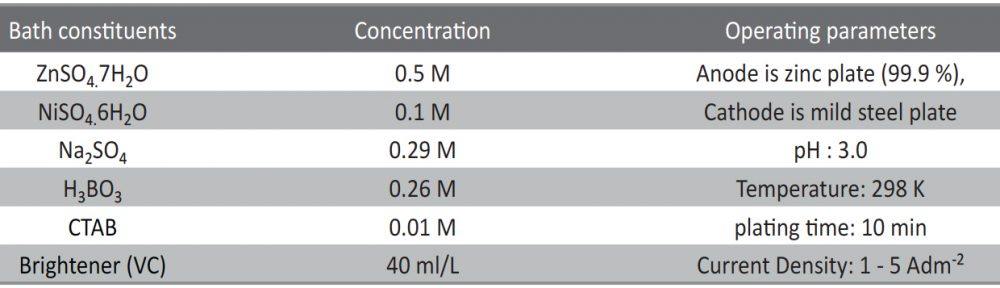
Electrochemical studies of the bright Zn-Ni alloy electrodeposit from acid sulphate bath
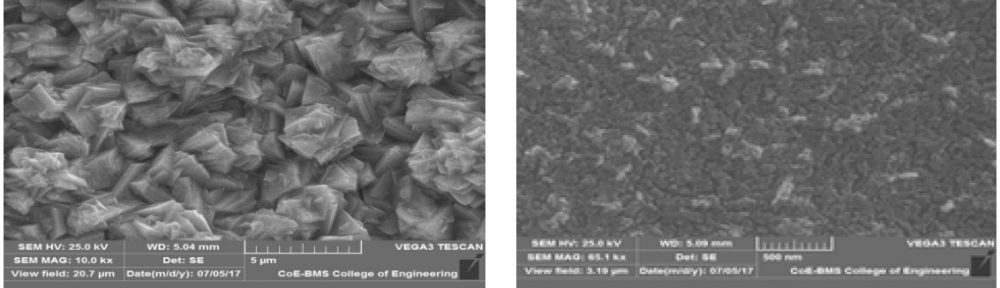
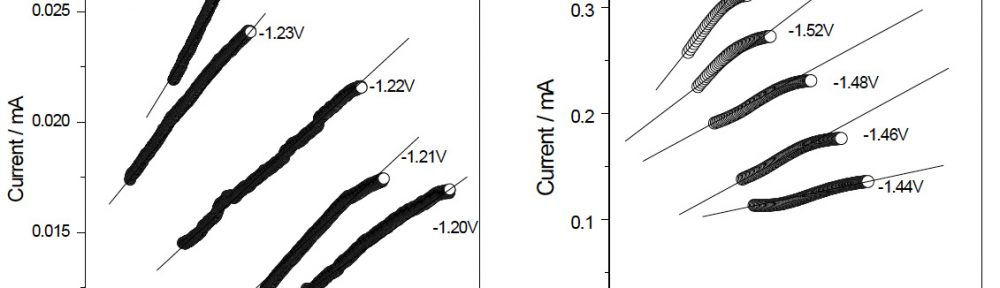
The condensation product of Vanillin and Cysteine Hydrochloride (VC) was used as an additive for the electrodeposition of Zn-Ni alloy on mild steel substrate. The bath constituents and operating conditions were optimized by Hull cell experiments. The electrochemical behaviour and nucleation mechanism was studied using cyclicvoltammetry and chronoamperometric techniques. The electrochemical studies revealed that electrocrystallisation process of zincnickel alloy coating was governed by three-dimensional (3D) nucleation process, controlled by diffusion. The model of Schariffker and Hills was used to analyze the current transients and it revealed that, in bright zinc-nickel alloy coating, the electrocrystallization process is regulated by instantaneous nucleation mechanism. The electrochemical impedance spectroscopy and Tafel polarization studies were used to study corrosion nature of Zn-Ni electrodeposits. Corrosion studies showed an improved corrosion resistant nature of bright Zn-Ni alloy coatings on mild steel substrate. The scanning electron microscopy (SEM) and X-ray diffraction (XRD) studies depicted smooth, compact and fine-grained structure of Zn-Ni electrodeposit in presence of VC, in plating bath solution.
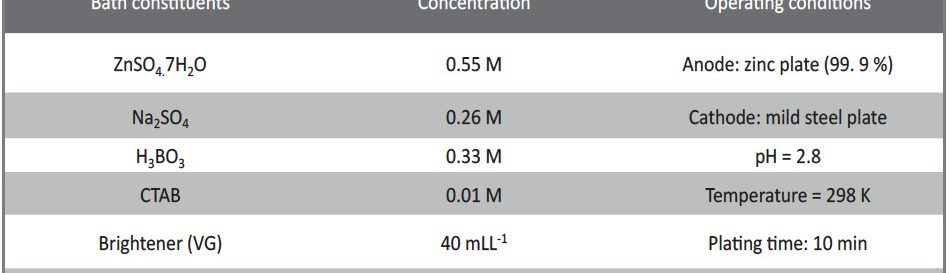
A study into the effect of a new brightener on electrodeposition and corrosion resistance of zinc
The electrodeposition of zinc on steel was obtained from an acid sulphate bath containing condensation product formed between Vanillin and Glycine (VG). The bath constituents and operating parameters were standardized by Hull cell experiments. The investigation of electrodeposition and nucleation mechanism was carried out on graphite electrode using cyclic voltammetric and chronoamperometric techniques. The corrosion studies were carried out by Polarisation and Electrochemical impedance techniques, which helped to explore the good protection ability of the zinc coating in presence of VG. The surface morphology of the deposit was characterised by scanning electron microscopy. Increase in brightness of the zinc coating obtained on mild steel substrate was confirmed by reflectance studies. The phase structure and the preferred orientation of the zinc crystallites were studied by X-ray diffraction analysis. These studies revealed the influence of VG in enhancing the brightness and corrosion resistance of the zinc electrodeposit on mild steel substrate.
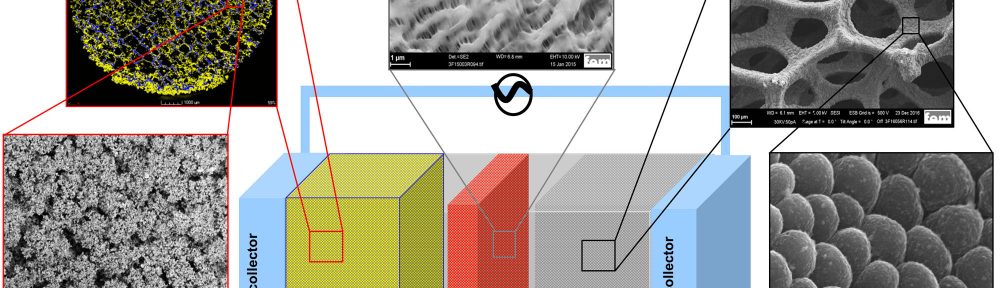
Electrodeposited Dendrite-Free, Nano-Columnar 3D Lithium Anodes and Their Application in Lithium Sulfur Batteries with 3D Sulfur Cathodes
In this work, homogeneous, dendritefree, nano-columnar lithium electrodeposition on 3D nickel foam from a 1 M lithium hexafluorophosphate (LiPF6) propylene carbonate (PC) electrolyte under convection has been performed successfully. The surface morphology and the thickness of the deposition can be varied depending on the electrodeposition parameters. In this way, it is possible to improve the surface area and reduce the amount of lithium in the cell, e.g. to only 50 % lithium excess, increasing cell safety. The new lithium plated 3D anode was developed to be combined with a 3D composite electroplated sulfur cathode, ensuring low local current densities at both, cathode and anode, which lowers overpotentials and therefore increase the cell efficiency. Furthermore, the porous electrodes can accommodate a larger amount of electrolyte, which is beneficial for an increased cycling stability. The results show that despite the reduction of lithium weight by a factor of 12 compared to a battery with a commercial 1.5 mm thick 2D lithium foil anode, the overall battery capacity on cell level, using cathodes with equal sulfur content, could even be improved.
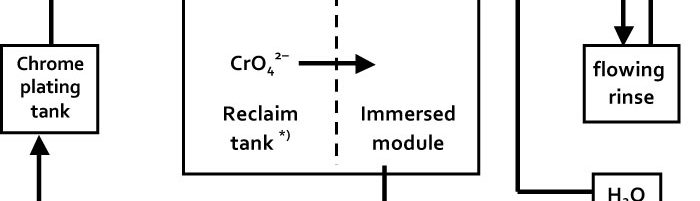
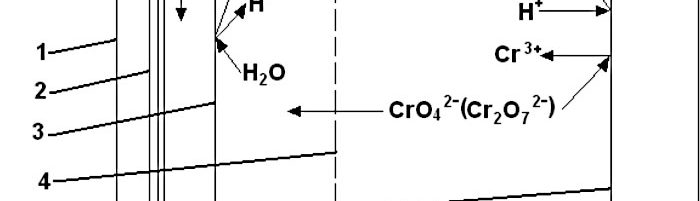
The Use of Immersed Electrochemical Modules in Plating Shops for the Regeneration of Process Solutions and Purification of Water in Reclaim Tanks
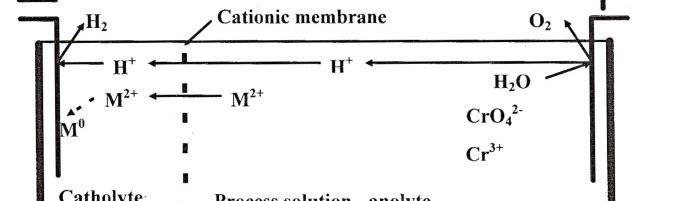
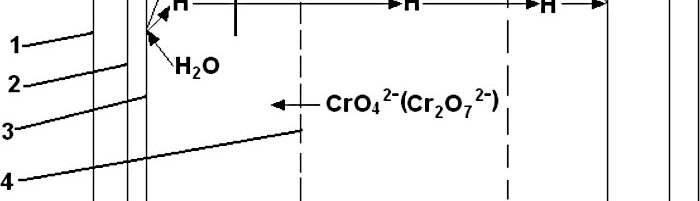
Immersed electrochemical module (IEM) is an electrochemical half-cell with one or two ion-exchange membranes and an inner electrode. IEM is immersed directly into a tank with a process solution in order to produce certain changes in its composition, for example, to recover nickel ions from spent electroless nickel plating solutions. Another area of application is to maintain the stable composition of process solutions such as various etchants used in the manufacture of PCBs, stripping and passivating solutions based on chromic acid and its salts. Stabilizing is achieved by anodic regeneration of an oxidant (chromate, ferric, cupric or persulfate ions) which are consumed in the course of the operation of the solution, by removing accumulating reaction products (various metal ions) and maintaining desirable pH value in the process solution. Continuous operation of such modules allows to eliminate periodic dumping and to reduce considerably consumption of chemicals used for replenishments. IEMs are used in many plating shops for continuous regeneration of chromate-based zinc passivating solutions. Another area of application of IMF is a continuous purification of water in reclaim tanks which allows to reduce the consumption of fresh water for rinsing and the amount of waste water. Metals such as zinc, copper, cadmium and tin are recovered from reclaim tanks equipped with IEMs and are usually returned into plating tanks. Nickel metal is utilized in some other way. Chromic acid which is recovered from reclaim tanks with IEMs contains no cationic impurities. It is returned into chromium plating or passivating process solutions. The operation of IEMs in reclaim tanks after chromium plating, anodizing or passivating in chromate-containing solutions allows to reduce the consumption of chemicals and the amount of waste. Installation of IEM does not need any additional floor space, pipe lines, etc. They are especially effective in chromating tanks and small-scale cadmium plating lines, where their use can solve problems related with the environment protection. IEMs are used in Russia in many captive plating shops.