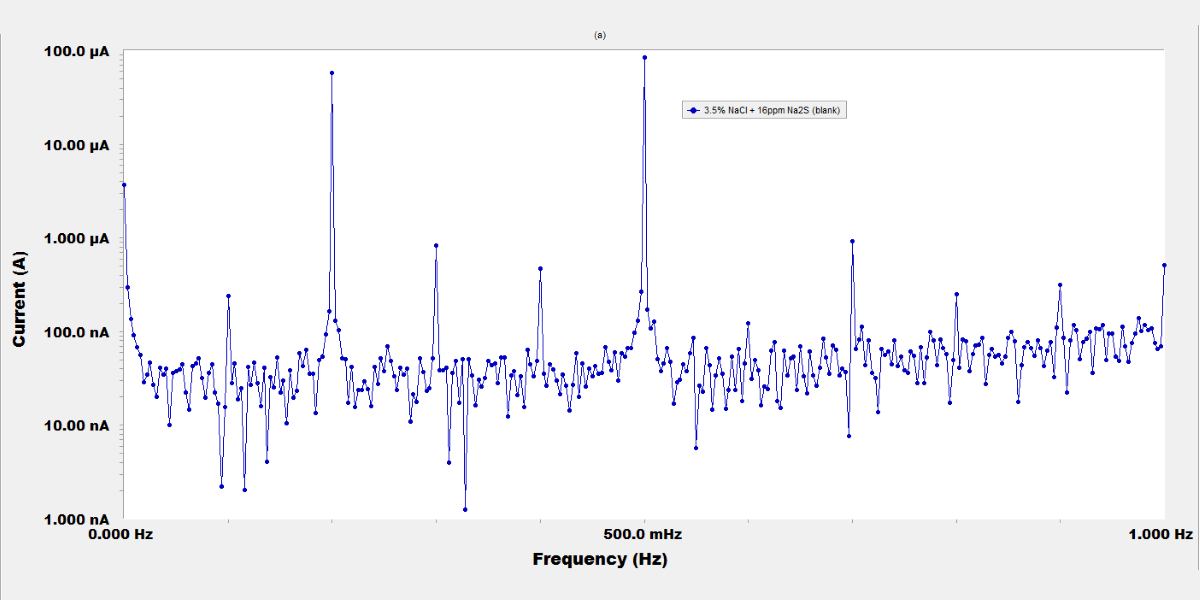
The inhibiting effect of molybdate, chromate, and tungstate salts on the corrosion of steel used in sanitation plants was investigated by potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), and electrochemical frequency modulation (EFM) techniques. The sulfide polluted salt water was simulated by a 3.5 % NaCl and 16 ppm Na2S solution. The results revealed that these inorganic anions are very good inhibitors. Potentiodynamic polarization curves indicated an anodic-type inhibition. The increased concentration of inorganic compounds increases the inhibition; at 250 ppm concentration the inhibition efficiency reached to 95 %, 91.6 %, and 87.5 % for MoO42-, CrO42-, and WO42-, respectively. The adsorption of the inhibitors on the metal surface basically obeys the Langmuir adsorption isotherm equation. Results of EIS measurements suggested that the dissolution of the steel occurs under activation control, and a passive film is probably formed on the metal surface. The electrochemical kinetic parameters calculated from EFM spectra confirm the polarization data.
