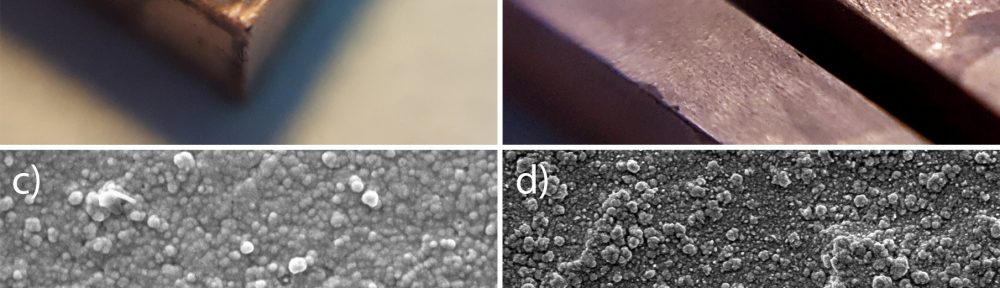
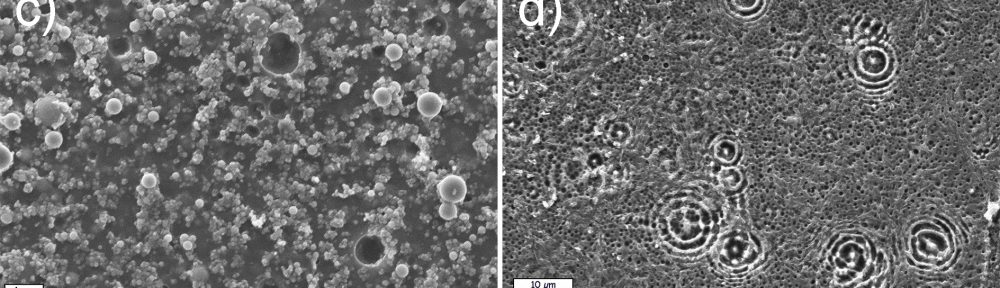
In the present paper, the influence of different wet pretreatment routes on the final quality of electroless layers plated on stereolithography resins is investigated. Two pretreatment methods, acidic and alkaline, are employed. Acidic etching is unable to provide acceptable results in terms of surface quality. On the contrary, alkaline etching guarantees a bright copper surface coupled with a level of adhesion high enough to pass a standard peel test. ATR FT-IR is employed to investigate the chemical reactions occurring on the resins during the pretreatment, evidencing a major role of the ester hydrolysis process on the depolymerization of the material and on the formation of new functional groups on the surface. Resin hydrolysis is linked with increase in surface roughness and wettability, parameters that strongly determine final metal adhesion. The combination of ATR FT-IT, contact angle and roughness measurement constitutes a possible combined methodology to follow the evolution of surface pretreatment on different stereolithography resins.
Electroforming with Nickel
While there are a large number of written documents on nickel as a coating, there are very few dealing with nickel electroforming. This technology is an important consumer of nickel – about 5,000 tons per year worldwide – used to manufacture a wide range of articles (it is a manufacturing process, not a coating process). It is indeed the ONLY process that can produce certain very important products that are very often used in society. Most people are not aware of the process and its importance. It is a process that is indispensable for the production of compact audio discs and digital visual discs. Since the annual production of these items is estimated at about 4.000,000,000, you can see how important it is for our lifestyle today. Another very large application is the mass production of holograms.
Diverse Applications of Simultaneous Occurrence of Oxidation-Reduction Reactions
Oxidation-reduction reactions also known as redox reactions for a shorthand term are typified by any chemical process that involves transfer of electrons between two species. The ubiquitous nature of redox reactions occurs over diverse systems ranging from industrial processes to chemical reactions encountered in everyday basic life functions. Based primarily on electrochemistry, the present article reviews the science of redox reactions with applications in some specific industrial and real life cases.
Electrochemical deposition of silicon from organic electrolytes
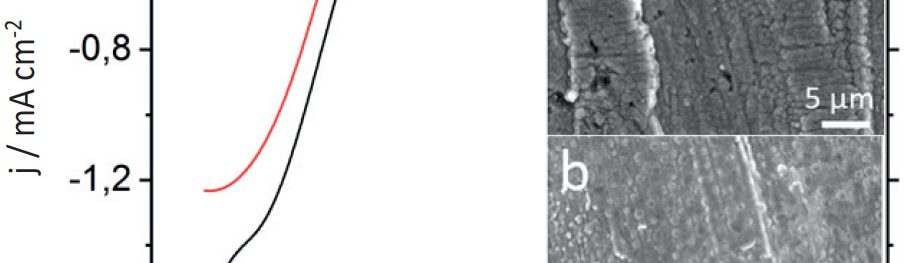
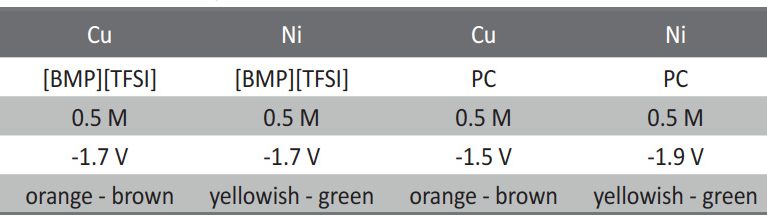
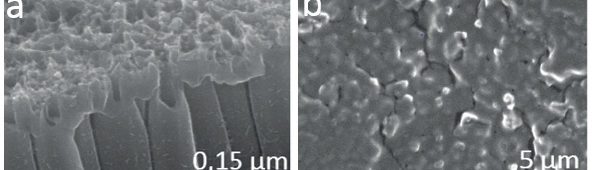
Electrochemical reduction of silicon from SiCl4 in 1-butyl-1-metyl-pyrrolidinium bis(trifluoromethylsulfonyl)imide [BMP][TFSI] and in propylene carbonate (PC) with SiCl4 as a precursor is performed at room temperature. The process is studied by means of Linear Sweep Voltammetry and chronoamperometry. The results exhibit considerable differences during the silicon deposition for copper and nickel. Scanning Electron Microscopy (SEM) of the layers shows a rough surface morphology. The composition of Si deposit is confirmed by Energy Dispersive X-ray analysis (EDX). Furthermore, the deposition of silicon onto TiO2 nanotubes is discussed. In conclusion, a method of recycling the used ionic liquid by a simple extraction procedure is presented.
Electrochemical studies of the bright Zn-Ni alloy electrodeposit from acid sulphate bath
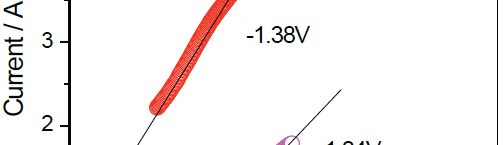
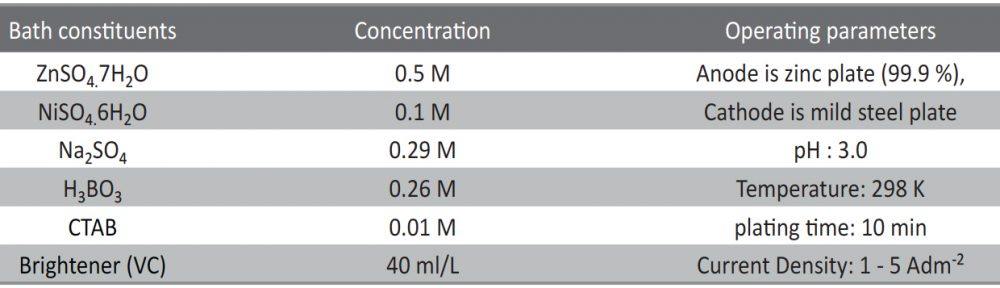
The condensation product of Vanillin and Cysteine Hydrochloride (VC) was used as an additive for the electrodeposition of Zn-Ni alloy on mild steel substrate. The bath constituents and operating conditions were optimized by Hull cell experiments. The electrochemical behaviour and nucleation mechanism was studied using cyclicvoltammetry and chronoamperometric techniques. The electrochemical studies revealed that electrocrystallisation process of zincnickel alloy coating was governed by three-dimensional (3D) nucleation process, controlled by diffusion. The model of Schariffker and Hills was used to analyze the current transients and it revealed that, in bright zinc-nickel alloy coating, the electrocrystallization process is regulated by instantaneous nucleation mechanism. The electrochemical impedance spectroscopy and Tafel polarization studies were used to study corrosion nature of Zn-Ni electrodeposits. Corrosion studies showed an improved corrosion resistant nature of bright Zn-Ni alloy coatings on mild steel substrate. The scanning electron microscopy (SEM) and X-ray diffraction (XRD) studies depicted smooth, compact and fine-grained structure of Zn-Ni electrodeposit in presence of VC, in plating bath solution.