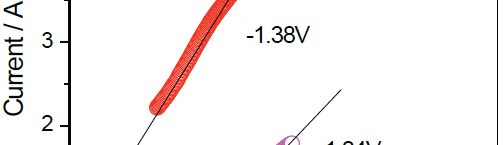
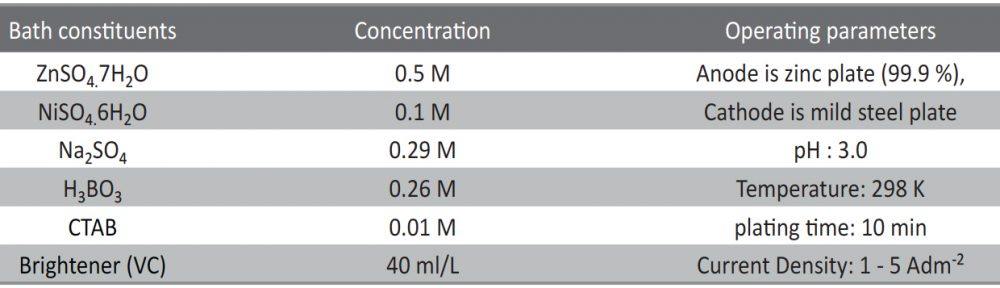
The condensation product of Vanillin and Cysteine Hydrochloride (VC) was used as an additive for the electrodeposition of Zn-Ni alloy on mild steel substrate. The bath constituents and operating conditions were optimized by Hull cell experiments. The electrochemical behaviour and nucleation mechanism was studied using cyclicvoltammetry and chronoamperometric techniques. The electrochemical studies revealed that electrocrystallisation process of zincnickel alloy coating was governed by three-dimensional (3D) nucleation process, controlled by diffusion. The model of Schariffker and Hills was used to analyze the current transients and it revealed that, in bright zinc-nickel alloy coating, the electrocrystallization process is regulated by instantaneous nucleation mechanism. The electrochemical impedance spectroscopy and Tafel polarization studies were used to study corrosion nature of Zn-Ni electrodeposits. Corrosion studies showed an improved corrosion resistant nature of bright Zn-Ni alloy coatings on mild steel substrate. The scanning electron microscopy (SEM) and X-ray diffraction (XRD) studies depicted smooth, compact and fine-grained structure of Zn-Ni electrodeposit in presence of VC, in plating bath solution.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany