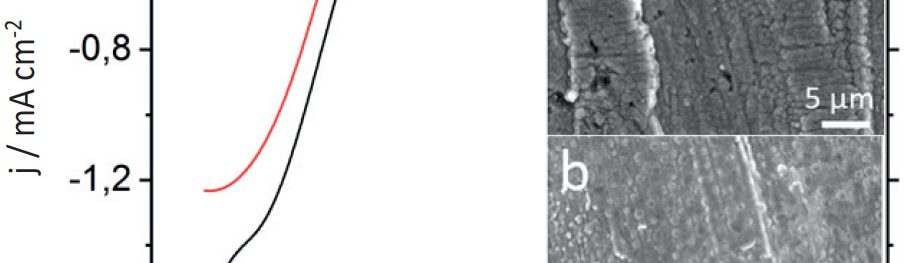
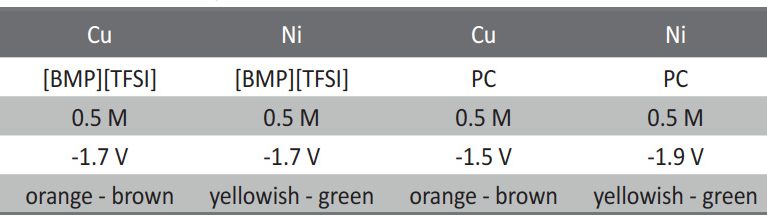
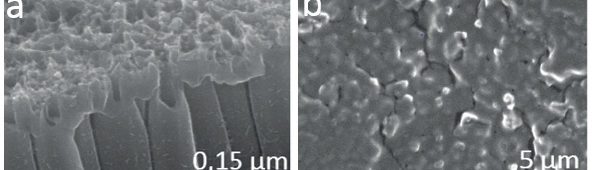
Electrochemical reduction of silicon from SiCl4 in 1-butyl-1-metyl-pyrrolidinium bis(trifluoromethylsulfonyl)imide [BMP][TFSI] and in propylene carbonate (PC) with SiCl4 as a precursor is performed at room temperature. The process is studied by means of Linear Sweep Voltammetry and chronoamperometry. The results exhibit considerable differences during the silicon deposition for copper and nickel. Scanning Electron Microscopy (SEM) of the layers shows a rough surface morphology. The composition of Si deposit is confirmed by Energy Dispersive X-ray analysis (EDX). Furthermore, the deposition of silicon onto TiO2 nanotubes is discussed. In conclusion, a method of recycling the used ionic liquid by a simple extraction procedure is presented.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany