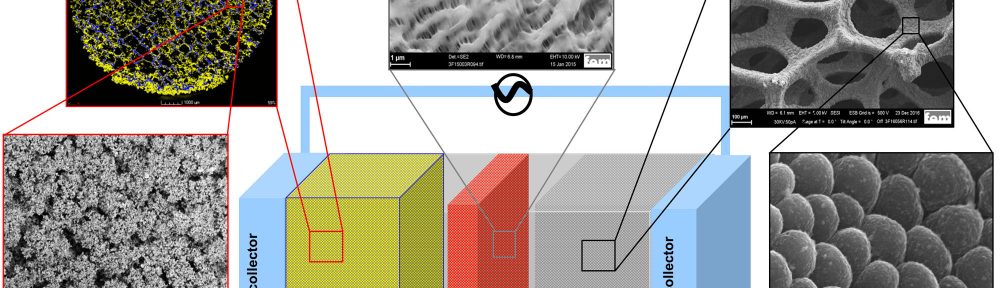
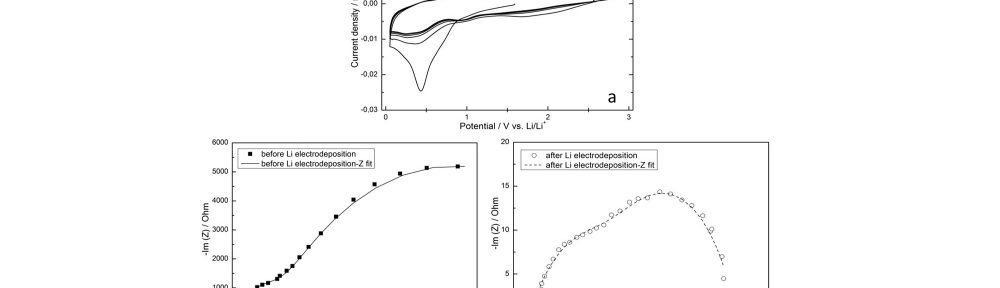
In this work, homogeneous, dendritefree, nano-columnar lithium electrodeposition on 3D nickel foam from a 1 M lithium hexafluorophosphate (LiPF6) propylene carbonate (PC) electrolyte under convection has been performed successfully. The surface morphology and the thickness of the deposition can be varied depending on the electrodeposition parameters. In this way, it is possible to improve the surface area and reduce the amount of lithium in the cell, e.g. to only 50 % lithium excess, increasing cell safety. The new lithium plated 3D anode was developed to be combined with a 3D composite electroplated sulfur cathode, ensuring low local current densities at both, cathode and anode, which lowers overpotentials and therefore increase the cell efficiency. Furthermore, the porous electrodes can accommodate a larger amount of electrolyte, which is beneficial for an increased cycling stability. The results show that despite the reduction of lithium weight by a factor of 12 compared to a battery with a commercial 1.5 mm thick 2D lithium foil anode, the overall battery capacity on cell level, using cathodes with equal sulfur content, could even be improved.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany