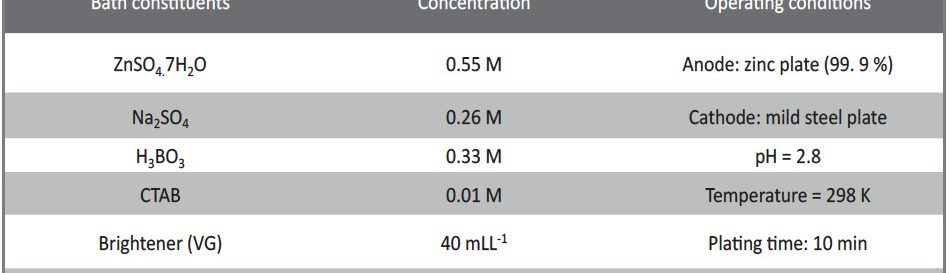
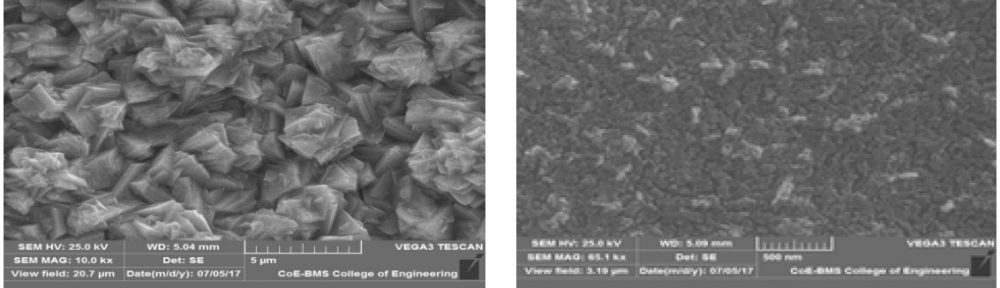
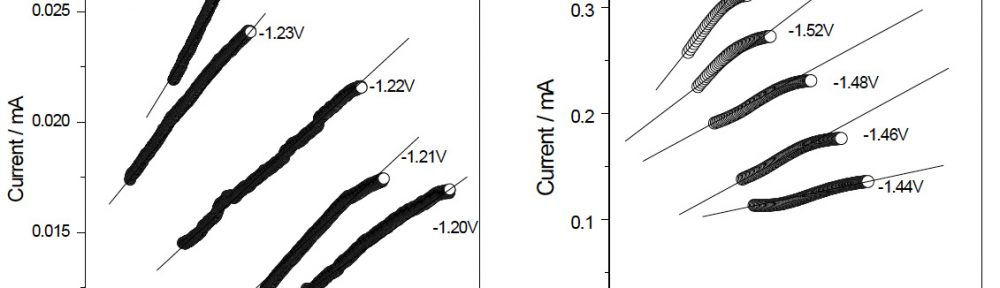
The electrodeposition of zinc on steel was obtained from an acid sulphate bath containing condensation product formed between Vanillin and Glycine (VG). The bath constituents and operating parameters were standardized by Hull cell experiments. The investigation of electrodeposition and nucleation mechanism was carried out on graphite electrode using cyclic voltammetric and chronoamperometric techniques. The corrosion studies were carried out by Polarisation and Electrochemical impedance techniques, which helped to explore the good protection ability of the zinc coating in presence of VG. The surface morphology of the deposit was characterised by scanning electron microscopy. Increase in brightness of the zinc coating obtained on mild steel substrate was confirmed by reflectance studies. The phase structure and the preferred orientation of the zinc crystallites were studied by X-ray diffraction analysis. These studies revealed the influence of VG in enhancing the brightness and corrosion resistance of the zinc electrodeposit on mild steel substrate.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany