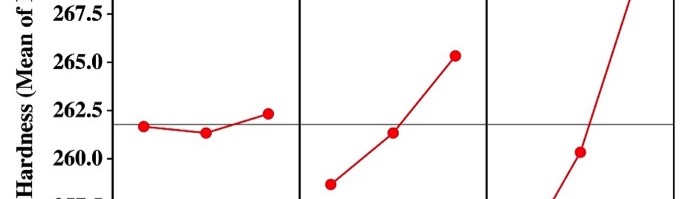
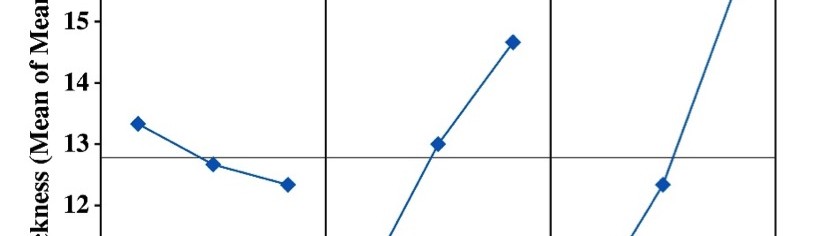
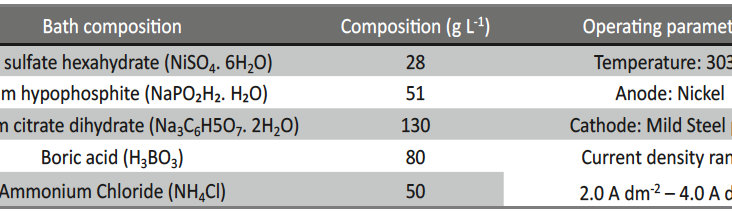
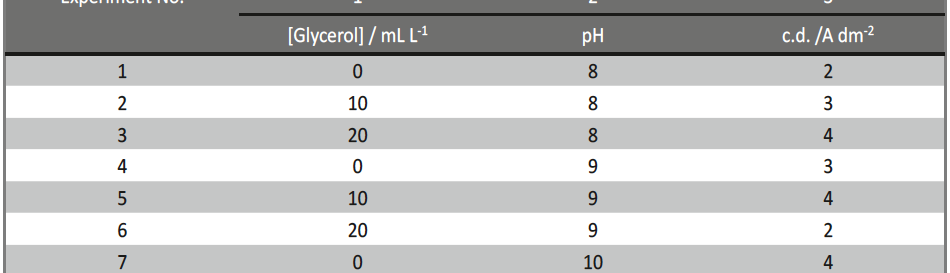
Incredible claims of electroplating in materials synthesis lies in tailoring its property by proper modulation of the bath composition and operating parameters, such as current density (c.d.), pH and temperature. Electroplating of metals/ alloys is one of the most complex process because of the unusually large number of critical elementary phenomena involved during deposition. Due to lack of quantitative guiding principles to develop a coating of desired property, it is very difficult and time consuming to optimize the bath composition. Even though Hull Cell method is an established method to optimize a bath, in terms of its constituents and operating parameters its application is limited to know only the effect of c.d. on deposit patterns; and is incapable for predicting the desired properties of the coating, like hardness, reflectivity, thickness etc. In this direction, this paper describes Taguchi’s statistical method for optimization of deposition conditions of Ni-P alloy, using Minitab 16, Statistical software, by reducing the number of experiments to a practical level. In the present study, bath variables, i.e., [glycerol], c.d. and pH of the bath are taken as chosen parameters and micro-hardness and thickness of the coatings as parameters for characteristic performance. Experimental conditions were optimized to maximize the coating properties. Taguchi’s method demonstrated that the basic Ni-P bath, having [glycerol] = 20 mL L-1, c.d.= 4.0 A dm-2 and pH = 8.0 as ideal for developing coatings of highest micro-hardness and thickness. Experimental data revealed that both [glycerol] and c.d. have close dependency on thickness and micro-hardness of coating, compared to pH of the solution. The experimental steps followed for applying Taguchi’s method, for tailoring the deposit characters are discussed with Tables and Figures.
Author Archives: Prof. Dr. A. Chitharanjan Hegde
Magnetic property and corrosion resistance of electrodeposited nanocrystalline cobalt-nickel alloys
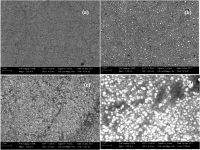
In the present investigation we have galvanostatically synthesized Co-Ni nanocrystalline alloys on copper substrate. The effect of current density (c.d.) on composition, surface morphology and phase structure were studied for explaining the magnetic and corrosion resistance of the alloy. The bath found to exhibit the preferential deposition of less noble Co than Ni, and at no conditions of c.d., the deposition has changed from anomalous to normal type. Surface morphology and structural characteristics of the deposits were examined using scanning electron microscopy (SEM) and X-ray diffraction (XRD) analysis. As composition of the alloy varied, consequent to the c.d. a change of hexagonal close packing structure (hcp) to face centered cubic structure (fcc) was observed. Finally, the conditions responsible for peak magnetic property and corrosion resistance were optimized. Factors responsible for improved functional properties were explained in terms of surface morphology and crystalline grain size of the coatings.
Electrodeposition and electrocatalytic performance of Ni-Co alloy
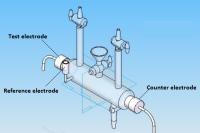
The electrodeposition of Ni-Co alloy coatings on pure copper has been carried out at different current densities (c.d.) from an aqueous sulphate bath at room temperature. The effects of c.d. on deposit characters such as composition, hardness and thickness have been studied. The electrodeposited Ni-Co coatings were tested for their electro-catalytic behaviors, namely for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in 6M KOH by cyclic voltammetry and chrono-potentiometry techniques. The surface morphology and phase structure of the deposit corresponding to different c.d.s were studied using, respectively FEGSEM and XRD study. The chrono-potentiometry study revealed that Ni-Co alloy coating deposited at 4.0 Adm-2 is more electro active for HER and that deposited at 1.0 Adm-2 is more electro-active for OER. Hence, Ni-Co alloy coatings deposited at 4.0 Adm-2 and 1.0 Adm-2 can be used as efficient electrode materials for, respectively HER and OER reactions finds applications in fuel cells as demonstrated by cyclic voltammetry (CV) and chrono-potentiometry experiments. The characteristic electro catalytic behaviour of the coatings for HER and OER are attributed to the inherent phase structure, composition, specific surface area and porosity of the coated materials under test, determined by the cathode current density at which they are deposited, supported by FEGSEM and XRD study.
Effect of additives and operating parameters on deposit characters of Ni-Cd alloy
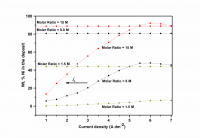
The Ni-Cd alloy coating was electrodeposited on mild steel (MS) from acid chloride bath using gelatin and glycerol as additives, individually and in combination. The bath composition and operating parameters have been optimized by conventional Hull cell method. The effect of current density (c.d.) on Ni content of the alloy was studied at different molar ratio of metal ions in the bath. The effects of c.d. and temperature on thickness, hardness, and composition and corrosion rate (CR) of the coatings were studied. Cyclic voltammetry (CV) study showed that (gelatin + glycerol) has significant effect on process of deposition and (gelatin + glycerol) worked synergistically to increase the Ni content by their preferential deposition and by suppressing the deposition of more readily depositable Cd2+ ions. Ni-Cd bath having both [Ni2+]/[Cd2+] = 1.5 and 8.0 exhibited anomalous type of codeposition at all c.d.’s studied. Corrosion behavior of the coatings evaluated by electrochemical methods demonstrated that the coating from bath [Ni2+]/[Cd2+] = 15, deposited at 4.0 A dm-2 is the most corrosion resistant. The superior corrosion resistance of Ni-Cd coatings at optimal c.d. was attributed to specific Ni (111), Ni (200), Cd (200) and Ni-Cd (862) reflections, evidenced by XRD study. The surface morphology was analyzed using SEM study, and results are discussed.
Electrofabrication of Composition Modulated Multilayer Alloy (CMMA) of Co-Ni for Better Corrosion Protection
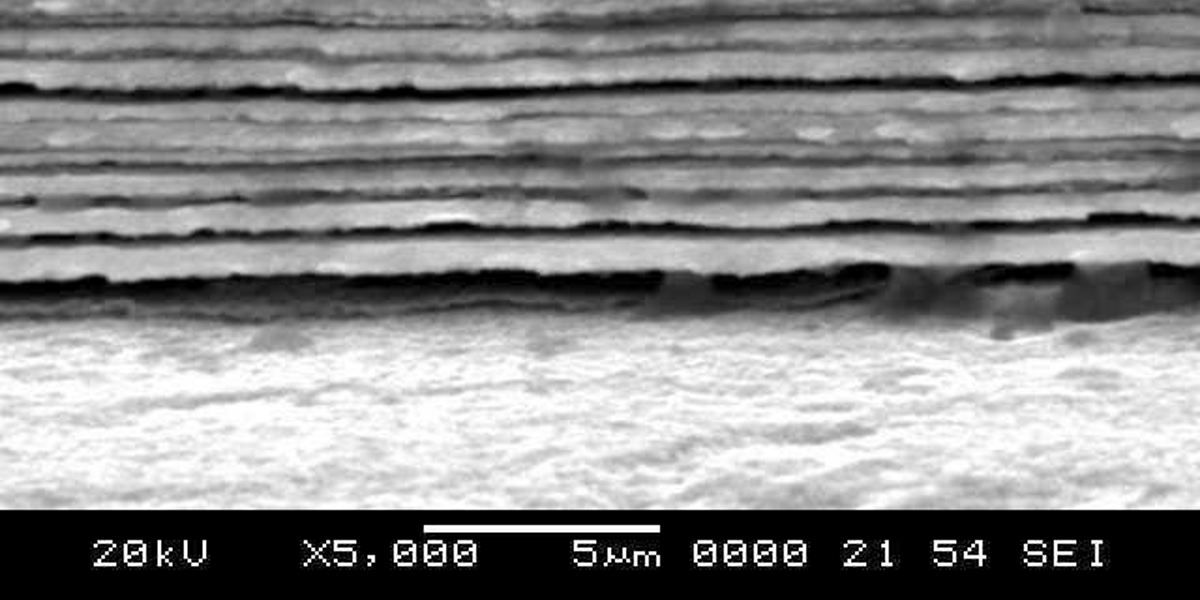
Electrofabrication of multilayer Co-Ni alloy coatings were accomplished successfully on mild steel (MS) for better corrosion protection. Multilayer comprised of alternatively formed ‘nano-size’ layers of Co-Ni alloy of different composition have been produced from single electrolyte having Co+2 and Ni+2 ions using modulated (i.e. periodic pulse control) current density. The deposition conditions were optimized for both composition and thickness of individual layers for best performance against corrosion. The process and product of depositions were analyzed using cyclic voltammetry and SEM, PXRD, Hardness Tester, electrochemical AC and DC methods, respectively. The corrosion behavior of multilayer coatings was found to be improved drastically when the thickness of individual layer approached nano regime. The coating having 300 layers, deposited at cyclic cathodic current densities of 2.0 and 4.0 Adm-2 was found to show the least corrosion rate (CR = 0.02 mmpy) compared to monolayer (Co-Ni)4 alloy coating (CR = 2.8 mmpy) deposited from the same bath for same deposition time. Drastic improvement in the corrosion performance of multilayer coatings were explained in the light of changed kinetics of mass transfer at cathode and increased surface area due to layering, respectively.