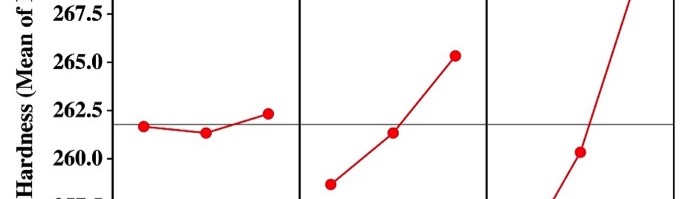
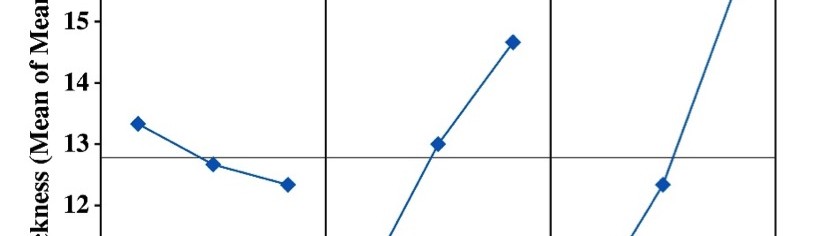
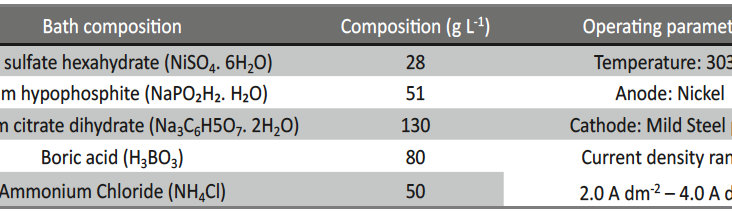
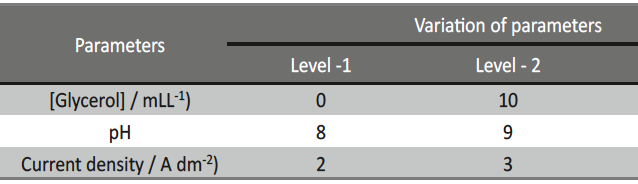
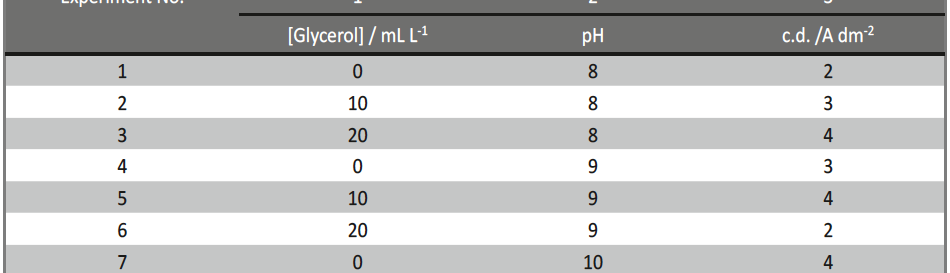
Incredible claims of electroplating in materials synthesis lies in tailoring its property by proper modulation of the bath composition and operating parameters, such as current density (c.d.), pH and temperature. Electroplating of metals/ alloys is one of the most complex process because of the unusually large number of critical elementary phenomena involved during deposition. Due to lack of quantitative guiding principles to develop a coating of desired property, it is very difficult and time consuming to optimize the bath composition. Even though Hull Cell method is an established method to optimize a bath, in terms of its constituents and operating parameters its application is limited to know only the effect of c.d. on deposit patterns; and is incapable for predicting the desired properties of the coating, like hardness, reflectivity, thickness etc. In this direction, this paper describes Taguchi’s statistical method for optimization of deposition conditions of Ni-P alloy, using Minitab 16, Statistical software, by reducing the number of experiments to a practical level. In the present study, bath variables, i.e., [glycerol], c.d. and pH of the bath are taken as chosen parameters and micro-hardness and thickness of the coatings as parameters for characteristic performance. Experimental conditions were optimized to maximize the coating properties. Taguchi’s method demonstrated that the basic Ni-P bath, having [glycerol] = 20 mL L-1, c.d.= 4.0 A dm-2 and pH = 8.0 as ideal for developing coatings of highest micro-hardness and thickness. Experimental data revealed that both [glycerol] and c.d. have close dependency on thickness and micro-hardness of coating, compared to pH of the solution. The experimental steps followed for applying Taguchi’s method, for tailoring the deposit characters are discussed with Tables and Figures.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany



Thank you for reading our article and giving good comments.