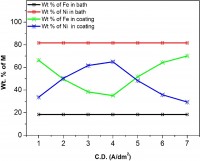
The electrodeposition of Ni-Co alloy coatings on pure copper has been carried out at different current densities (c.d.) from an aqueous sulphate bath at room temperature. The effects of c.d. on deposit characters such as composition, hardness and thickness have been studied. The electrodeposited Ni-Co coatings were tested for their electro-catalytic behaviors, namely for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in 6M KOH by cyclic voltammetry and chrono-potentiometry techniques. The surface morphology and phase structure of the deposit corresponding to different c.d.s were studied using, respectively FEGSEM and XRD study. The chrono-potentiometry study revealed that Ni-Co alloy coating deposited at 4.0 Adm-2 is more electro active for HER and that deposited at 1.0 Adm-2 is more electro-active for OER. Hence, Ni-Co alloy coatings deposited at 4.0 Adm-2 and 1.0 Adm-2 can be used as efficient electrode materials for, respectively HER and OER reactions finds applications in fuel cells as demonstrated by cyclic voltammetry (CV) and chrono-potentiometry experiments. The characteristic electro catalytic behaviour of the coatings for HER and OER are attributed to the inherent phase structure, composition, specific surface area and porosity of the coated materials under test, determined by the cathode current density at which they are deposited, supported by FEGSEM and XRD study.