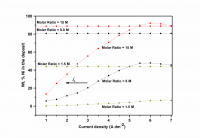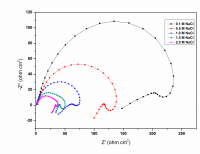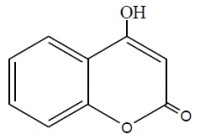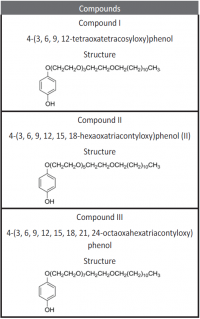
Inhibition of aluminum corrosion in 1M HCl in absence and presence of three compounds of non ionic surfactants compounds derived from phenol was investigated using hydrogen evolution reaction, weight loss galvanostatic polarization and electrochemical impedance spectroscopy techniques. It was found that the percentage inhibition increases with increasing the concentration of inhibitor, amount of ethylene oxide unit and with decreasing temperature. The inhibitive action of non ionic surfactant compounds was explained in terms of blocking the electrode surface by adsorption process. The adsorption process follows Langmuir isotherm. The polarization measurements showed that these inhibitors are acting as mixed inhibitors for both cathodic and anodic reaction. Electrochemical impedance spectroscopy technique exhibit one capacitive loop indicating that, the corrosion reaction is controlled by charge transfer process. Some activated thermodynamic parameters are calculated and explained.