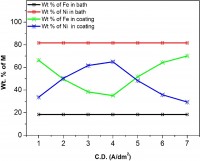
Nanocrystalline Fe-Ni coatings were electrodeposited on mild steel (MS) panels at different current densities (c.d.) from an acid sulphate electrolyte. The operating parameters were optimized for best appearance and performance of the coatings. Different techniques like Field Emission Gun Scanning Electron Microscopy (FESEM), Energy Dispersive X-ray Analysis (EDXA), X-Ray Diffraction, potentiodynamic polarization scan and Electrochemical Impedance Spectroscopy (EIS) were employed to characterize the electrodeposited thin films. The electrodeposition process was found to be anomalous with 35% to 70% Fe, depending on the current densities (c.d.) employed for deposition. The properties of all coatings were found to show close dependency with c.d., phase structure and composition of the alloys. Corrosion behaviors were studied in 5% HCl and 5% KOH medium and corrosion parameters were reported. Experimental results are discussed by relating the composition, phase structure and grain size with corrosion performance of the coatings in both acid and alkaline medium.