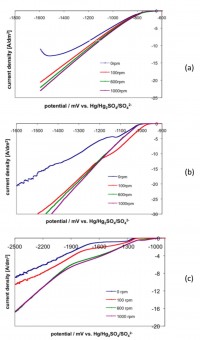
After having reached the 65th year of life in 2005, Prof. Dr. Siegfried Steinhäuser officially was officially retiring – but as adherent of TU Chemnitz he continues to work on his subject area (materials science) and engages i.a. in China. In September 2013 Prof. Steinhäuser was now honored with the Friendship Prize awarded by the Governor of Jiangsu Province, Mao Weiming, in the capital Nanjing. Jiangsu Province with its 80 million inhabitants counts among the most prospering provinces in China. Within a solemn act also other outstanding personalities from industry and academia, i.a. from Canada, India and Sweden, were honored. Continue reading…