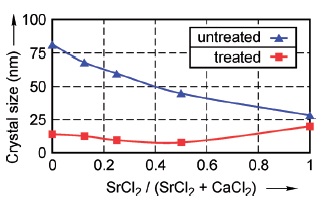
For the first time, strontium hydrogen phosphate (SrHPO4) was electrocrystallized on titanium substrate by means of electrochemical deposition technique, and converted to strontium hydroxyapatite (Sr10(PO4)6(OH)2) to improve implant adhesion and bone mineralization. Brushite (calcium phosphate dihydrate CaHPO4·2H2O) and strontium hydrogen phosphate were co-electrocrystallized on titanium substrate. With increasing SrCl2 and decreasing CaCl2 in the solution, Sr concentration in the coating was increased. Calcium substitution by strontium ranged from 0 to 100 atomic percent, thus having significant effect on layer thickness, morphology, and composition. Layers containing brushite and strontium hydrogen phosphate were converted to calcium hydroxyapatite and strontium hydroxyapatite. Strontium hydroxyapatite was formed in the case of 100 percent SrCl2 substituting CaCl2. Surface morphology, chemical composition, and phase identification of the coatings were studied by scanning electron microscopy combined with energy dispersive spectrometry (SEM-EDXS) and by X-ray diffractometry (XRD). Effects of the varying Sr substitution on the microstructure and properties are discussed.