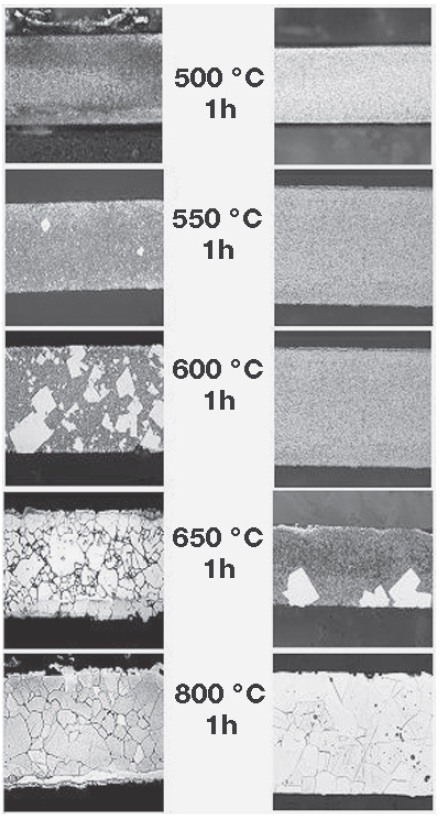
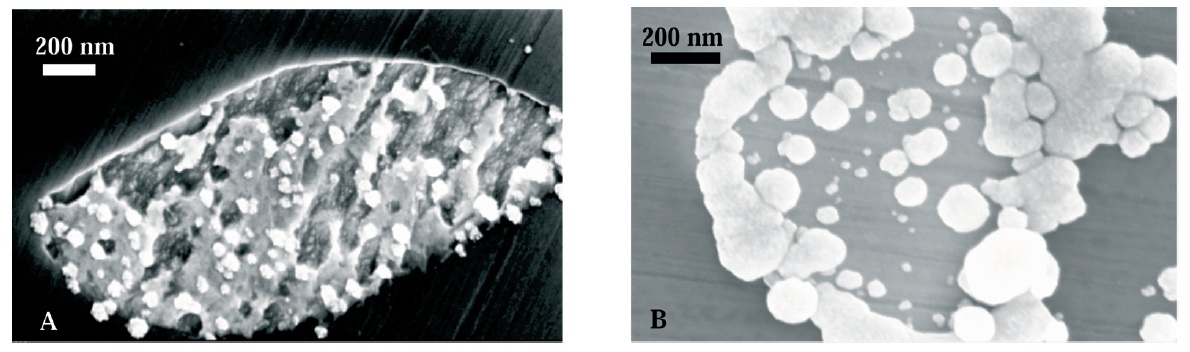
Results of electrocodeposition of gold matrix composite coatings with carbon-based materials are reported, namely ultradispersed diamonds (UDD) and multiwalled carbon nanotubes (MWCNT). Pure gold and gold composite coatings were prepared from a gold sulphite electrolyte with bath loads from 5 to 20 g/l (UDD bath) and from 0.1 to 5 g/l (MWCNT bath). The resulting composites are characterized in terms of carbon content, particle distribution, and their bonding to the matrix, surface morphology, and the influence of particle loading in the electrolyte on matrix microstructure. Vickers hardness, friction, and wear behavior were investigated and are discussed in terms of microstructure characterization. Some notable improvements in the performance of the composites were observed with regard to application as sliding contacts.