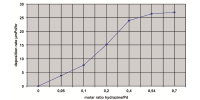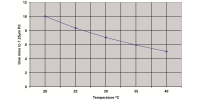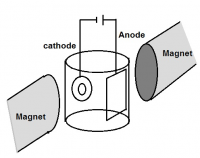
The electrodeposition of NiCo alloy has been investigated in presence of various magnetic fields. The influence of superimposed magnetic field (0-0.5T) parallel to the electrode surface on current efficiency, surface morphology, preferred crystal orientation and electrochemical activity of Ni-Co alloy were studied. The maximum current efficiency was obtained by direct current at 100mA/cm2 with 0.4T. The optimized current density (100mA/cm2) was pulsed at four different frequencies (10, 25, 50 and 100Hz) with the same magnetic field (0.4T). However, the superimposition of magnetic field significantly favors the preferred crystal orientation of (220) phase. Pulsed current deposits exhibit single orientation of (220) at lower magnetic field (0.4T) whereas direct current deposition require higher magnetic field (0.5T). Tafel plot shows that electro-catalytic activity and corrosion resistance property has improved when the deposit is having a preferred orientation of (220).