In the present work, nickel-based alloy coatings (Ni-Co, Sn-Ni and Ni-W) with different microstructures were produced under direct and pulse current conditions. These alloys are of interest as potential replacement for chromium based coatings, especially hard chromium. A replacement for hard chromium coating faces the challenge of providing sufficient hardness values. For some sliding wear applications, hardness might not be required at the same level as hard chromium, and coating toughness might be more critical.
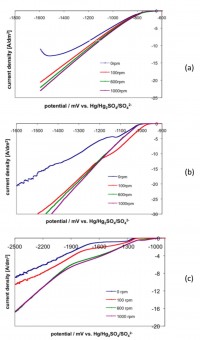
The effects of the pulse parameters (pulse waveform, pulse frequency and average current density) on the deposit structure and properties of these three systems have been investigated. Pulses have been defined based on the results of electrochemical measurements and numerical process simulation. The surface morphology, microstructure and microhardness of the deposit have been correlated to the pulse parameters applied.
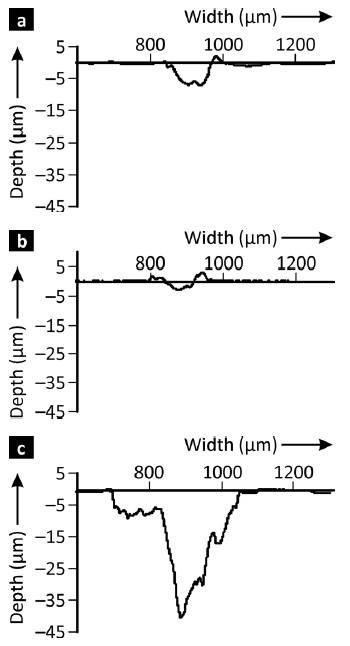
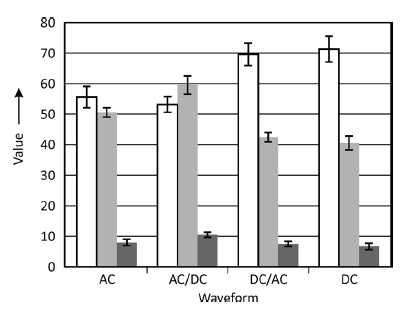
The experimental results showed that applying pulse plating substantially altered the properties of the coatings. The resulting layers exhibited a nano-crystalline microstructure, improved layer compactness and hardness of the nickel-based alloy deposits.