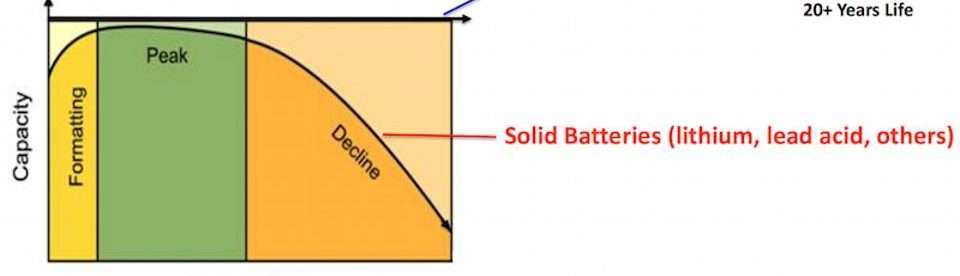
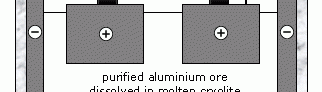
Oxidation-reduction reactions also known as redox reactions for a shorthand term are typified by any chemical process that involves transfer of electrons between two species. The ubiquitous nature of redox reactions occurs over diverse systems ranging from industrial processes to chemical reactions encountered in everyday basic life functions. Based primarily on electrochemistry, the present article reviews the science of redox reactions with applications in some specific industrial and real life cases.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany