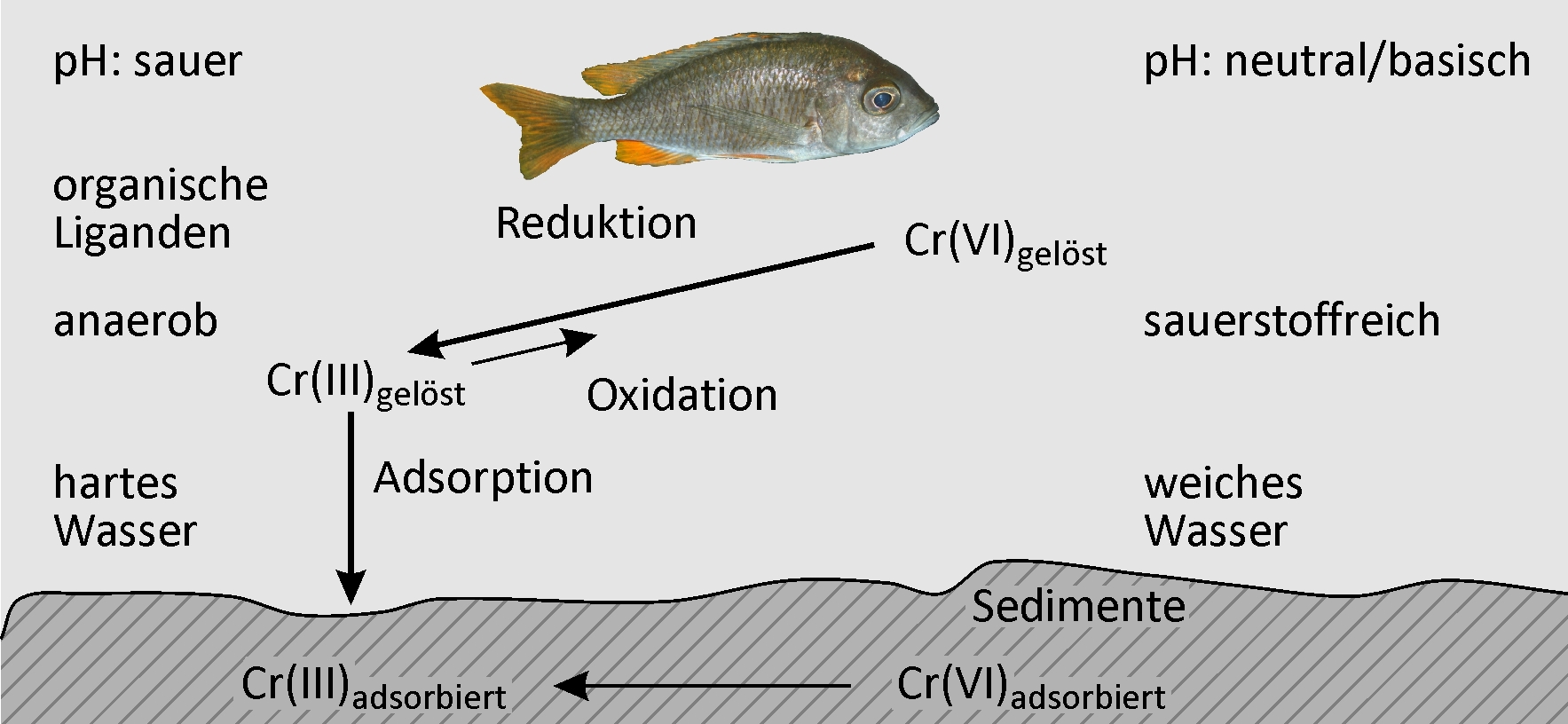
This article focuses on ecotoxicological properties of chromium(VI) compounds, their behavior in the environment, as well as consequences for safe handling such compounds. In natural environments, chromium is usually part of complex compounds. Therefore, higher concentrations of biologically available chromium compounds are mostly due to human activity. Trivalent and hexavalent chromium remain stable in natural environments. Both oxidation states are inter-convertible via redox reactions. As studies on organisms at all levels of the food chain show, hexavalent chromium is characterized by relatively high acute and chronic aquatic toxicity. From a regulatory point of view, this implies that additional risk management measures are necessary in order to reduce the input of chromium(VI) compounds into the environment as far as possible. At the same time, the properties of the substance call for an intensive search for substitute materials and processes that guarantee the functionality needed but cause less problems for human health and the environment. Further details are available in European Risk Assessment Report 53 [1].