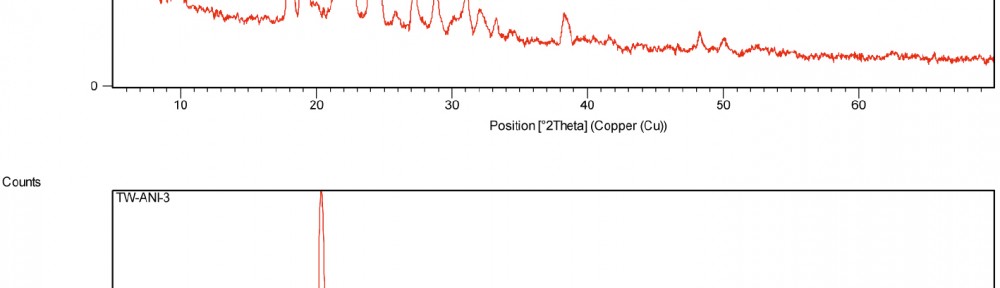
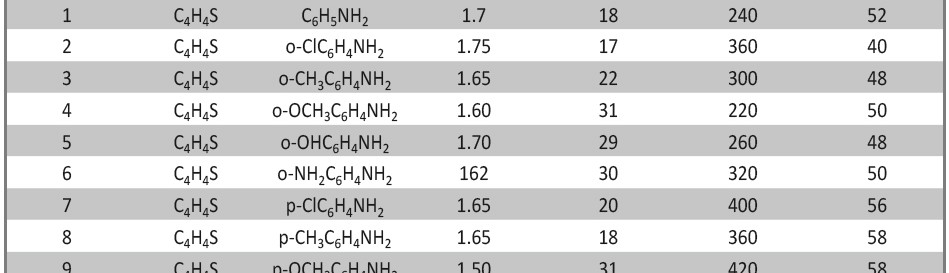
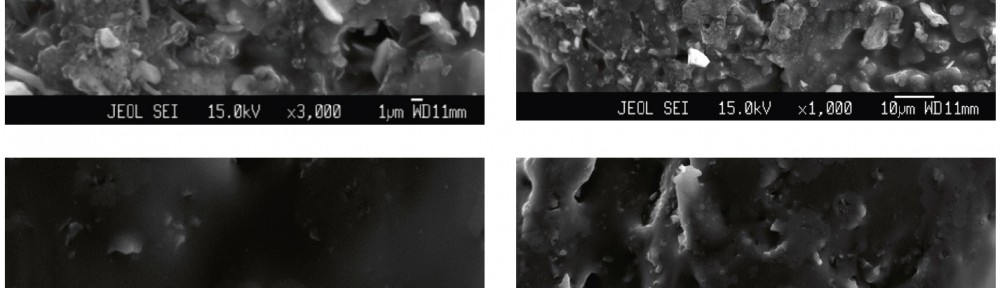
A new series of copolymers, obtained by reacting aniline as electron donor with thiophene as electron acceptor in a donor–acceptor structure (poly-thio-co-ani), were synthesized via electrochemical polymerization using acetonitrile as a solvent and lithium perchlorate as supporting electrolyte. The copolymer have better solubility in DMSO and KOH than the corresponding homopolymers. Copolymerization of aniline and thiophene was studied by UV-visible and FT-IR spectroscopy. In order to analyze their structure and characteristics X-ray diffraction analysis was applied and the samples were photographed under scanning electron microscope (SEM) for microstructure analysis and morphological property. Electrochemical properties were observed by cyclic voltammetry.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany