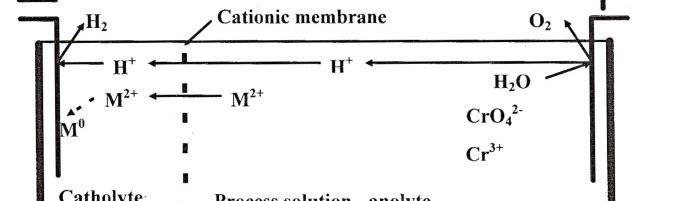
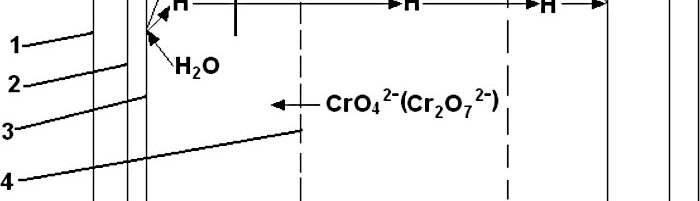
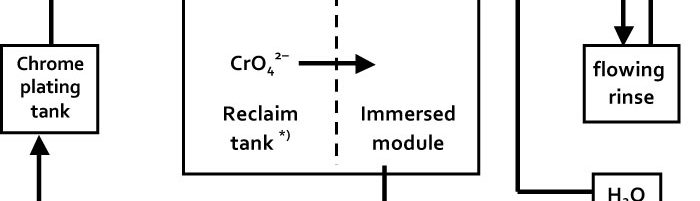
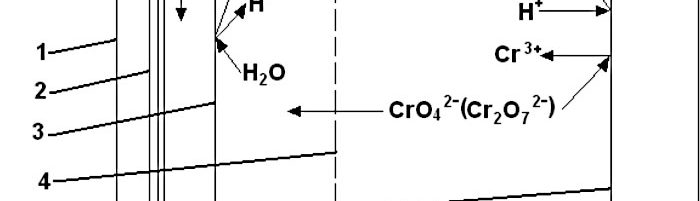
Immersed electrochemical module (IEM) is an electrochemical half-cell with one or two ion-exchange membranes and an inner electrode. IEM is immersed directly into a tank with a process solution in order to produce certain changes in its composition, for example, to recover nickel ions from spent electroless nickel plating solutions. Another area of application is to maintain the stable composition of process solutions such as various etchants used in the manufacture of PCBs, stripping and passivating solutions based on chromic acid and its salts. Stabilizing is achieved by anodic regeneration of an oxidant (chromate, ferric, cupric or persulfate ions) which are consumed in the course of the operation of the solution, by removing accumulating reaction products (various metal ions) and maintaining desirable pH value in the process solution. Continuous operation of such modules allows to eliminate periodic dumping and to reduce considerably consumption of chemicals used for replenishments. IEMs are used in many plating shops for continuous regeneration of chromate-based zinc passivating solutions. Another area of application of IMF is a continuous purification of water in reclaim tanks which allows to reduce the consumption of fresh water for rinsing and the amount of waste water. Metals such as zinc, copper, cadmium and tin are recovered from reclaim tanks equipped with IEMs and are usually returned into plating tanks. Nickel metal is utilized in some other way. Chromic acid which is recovered from reclaim tanks with IEMs contains no cationic impurities. It is returned into chromium plating or passivating process solutions. The operation of IEMs in reclaim tanks after chromium plating, anodizing or passivating in chromate-containing solutions allows to reduce the consumption of chemicals and the amount of waste. Installation of IEM does not need any additional floor space, pipe lines, etc. They are especially effective in chromating tanks and small-scale cadmium plating lines, where their use can solve problems related with the environment protection. IEMs are used in Russia in many captive plating shops.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany