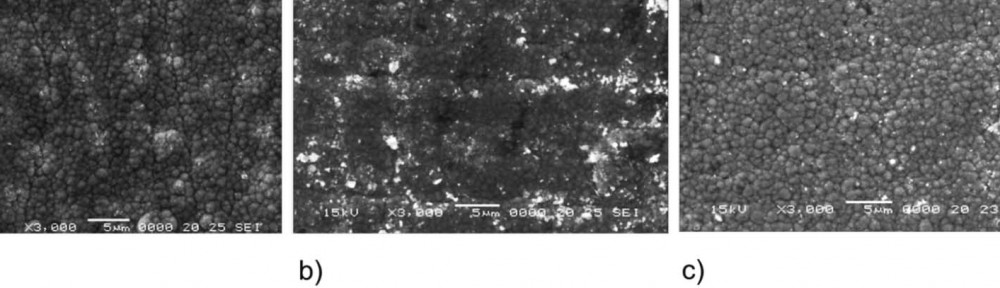
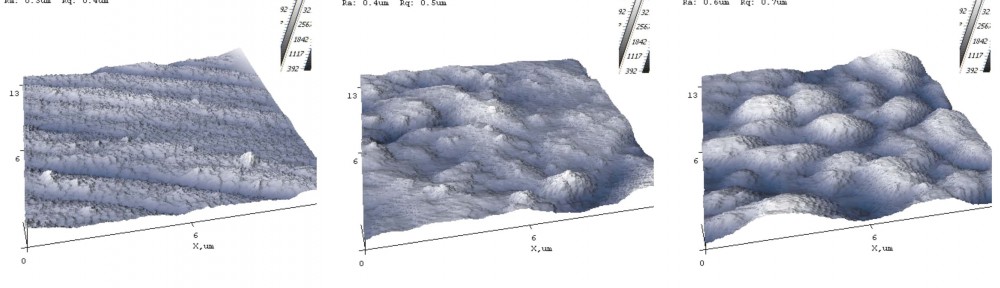
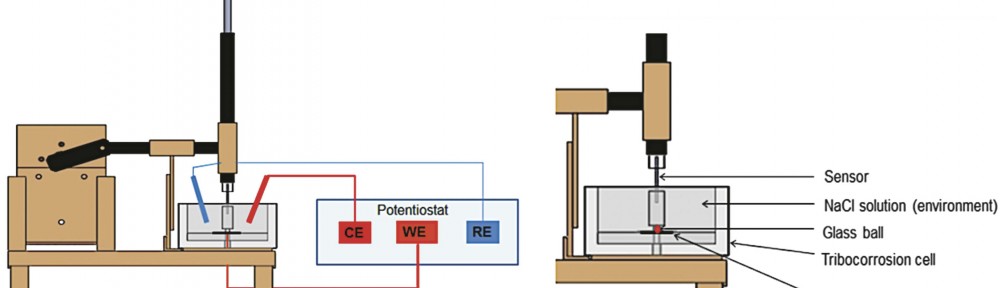
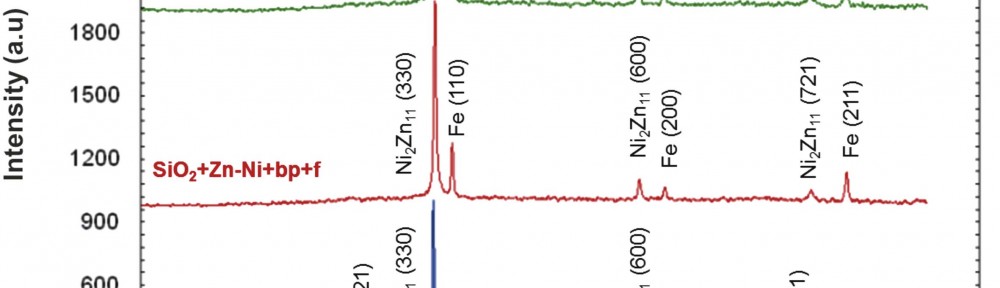
The tribological behavior of an electrodeposited Zn-Ni alloy layer has been investigated to understand the effect of substrate and electroplating methods. The substrates used were: steel, steel/silicon dioxide and steel/silicon dioxide/boron nitride. The tribological behavior was investigated using a ball-on-plate tribometer equipped with an electrochemical cell with 1 % NaCl solution. Open circuit potential measurements, chrono-amperometry (CA) (constant potential electrolysis technique – CPE) measurements and electro-chemical impedance spectroscopy (EIS) measurements were made, before and after a wear test. The coefficient of friction was also measured. The structure and morphology of the electrodeposited layers and the nature of the corrosion products were determined using SEM, XRD and AFM measurements. The Zn-Ni coating electrodeposited using pulse current electrodeposition on steel/silicon dioxide/boron nitride substrate was found to have a higher tribocorrosion resistance compared to the Zn-Ni layers electrodeposited by using pulse current electrodeposition and electrodeposition in a magnetic field on steel/silicon dioxide or by conventionally electrodeposited on steel substrate.
JEPT – Journal for Electrochemistry and Plating Technology
Edited by: DGO-Fachausschuss Forschung – Hilden / Germany